
(12��)
ʵ����������װ�òⶨFeO��Fe2O3����������Fe2O3��������Dװ�õ�Ӳ��˫ͨ�������еĹ���������FeO��Fe2O3�Ļ���

��1����μ��װ��A��������
��2��Ϊ�˰�ȫ���ڵ�ȼD���ľƾ���֮ǰ����b������ ��
(3)װ��B��������
װ��C��װ��Һ����
��4������������ã����ҽ����˱�Ҫ�İ�ȫ������ȼD���ľƾ��ƣ���Ӳ��˫ͨ�������з����Ļ�ѧ��Ӧ����ʽ��
��5����FeO��Fe2O3�������������Ϊ23. 2g����Ӧ��ȫ��U�ܵ���������7.2g��������Fe2O3������Ϊ _��
��1���֣�
��1��������������©���м�ˮ����ˮ���������²��İ�����ʱ���ر�������������ˮ��ʹˮ����������©���С�����Ƭ�̣���ˮ�治�½�����˵��װ������������
��2���鴿
��3����HCl��ŨH2SO4
��4��Fe2O3+3H2=2Fe+3H2O FeO+H2=Fe+H2O
��5��16g
���������������������ȡ�����ʵIJⶨ�����ʵ�����
��3��װ��B��CΪ����ľ���װ�ã��ֱ��ȥ�������HCl��ˮ������
��4�����ݻ���������������ˮ�������г���������㼴�ɡ�


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ʵ�����ü���l���������廯�ƺ�ŨH2SO4�Ļ����ķ������Ʊ�1���嶡��ʱ��������ϩ���ѵȸ��������ɡ���Ӧ������Ӧ�����������õ�1���嶡�飬��֪����л�����������£�
���Ʊ�1���嶡���װ��Ӧѡ����ͼ�е�_______������ţ�����Ӧ����ʱ���¶Ȳ��˳���
100�棬������_________________________��
���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ��� ��
�Ƿ�Ӧ��������Ӧ�������1���嶡����������Ӧѡ�õ�װ����______���ò���Ӧ���Ƶ��¶ȣ�t����Χ��_______________��
������ȥ������е���������Br2���������������ʺϵ��� ��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ�ͷ33�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(12��)
ʵ����������װ�òⶨFeO��Fe2O3����������Fe2O3��������Dװ�õ�Ӳ��˫ͨ�������еĹ���������FeO��Fe2O3�Ļ���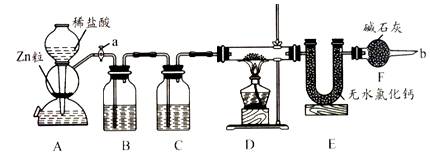
��1����μ��װ��A��������
��2��Ϊ�˰�ȫ���ڵ�ȼD���ľƾ���֮ǰ����b������ ��
(3)װ��B��������
װ��C��װ��Һ����
��4������������ã����ҽ����˱�Ҫ�İ�ȫ������ȼD���ľƾ��ƣ���Ӳ��˫ͨ�������з����Ļ�ѧ��Ӧ����ʽ��
��5����FeO��Fe2O3�������������Ϊ23. 2g����Ӧ��ȫ��U�ܵ���������7.2g��������Fe2O3������Ϊ _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�������и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(12��)ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣�ش��������⣺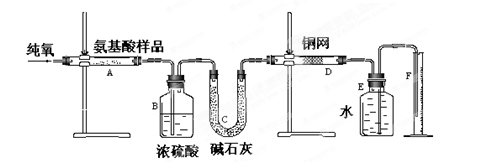
(1)ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������_ ��
(2)����װ������Ҫ���ȵ�������______(��д��ĸ)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________ ___________________��
(4)Dװ�õ������� ��
(5)ʵ���в�õ��������ΪV mL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������_ __��
| A�����ɶ�����̼��������� | B������ˮ������ |
| C��ͨ����������� | D�����������Է������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣�ش��������⣺
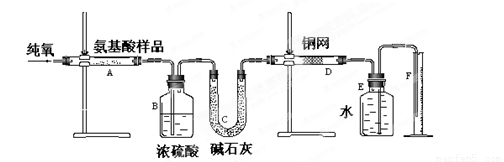
(1)ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������_ ��
(2)����װ������Ҫ���ȵ�������______(��д��ĸ)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________ ___________________��
(4)Dװ�õ������� ��
(5)ʵ���в�õ��������ΪV mL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������_ __��
A�����ɶ�����̼��������� B������ˮ������
C��ͨ����������� D�����������Է�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�������ѧ�߶���ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ�ʵ����
(12��)ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣�ش��������⣺
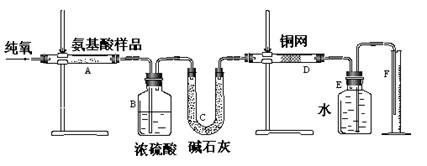
(1)ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������_ ��
(2)����װ������Ҫ���ȵ�������______(��д��ĸ)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________ ___________________��
(4)Dװ�õ������� ��
(5)ʵ���в�õ��������ΪV mL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������_ __��
A�����ɶ�����̼��������� B������ˮ������
C��ͨ����������� D�����������Է�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com