
���� ��1����������������Һ��Ӧ����ƫ��������������
��2��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��3�����ݷ�Ӧ��Ϊ���������Һ�����֣�Ҫ�õ����������Ҫ��ʵ��������
��4������Mg��Al���ܹ���������ѧ��Ӧ�����������������Ҫ֪�������ݣ�
��5���������þ���������ʵ��������ݺϽ�����������պ��������ʽ�����þ�����ʵ������ټ����þ������������
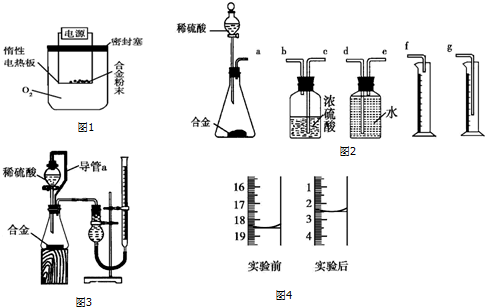
��6������װ�õ���װ˳�Ͻ����ᷴӦ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�������������
��7���ٱ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ�������������ע��Ӧ���ָ������ζ�����Һ��ȸߣ����ռ�������ζ�����Һ��������������С��
�۸���ϡ���ᶨ����ƿ�ܹ��������ų�������
��� �⣺��1����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����þΪ3%ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ��5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2 +3H2��
54g 2mol
5.4g��97% V��10-3L��2.0mol/L
����54g����5.4g��97%��=2mol����V��10-3L��2.0mol/L������ã�V=97����V��NaOH��Һ����97mL��
�ʴ�Ϊ��97��
��3����Ӧ���������ȫ���ģ�û�з�Ӧ��Ϊ����þ����������þ֮ǰ��Ҫ�������ˡ�ϴ�ӡ����������Ȼ���ٳ��������������Ӷ�������Ͻ���þ�ĺ�����
�ʴ�Ϊ�����ˡ�ϴ�ӡ�������壻
��4��Mg��Al����������Ӧ�����ɽ������������ⶨ�������������
�ʴ�Ϊ�����պ�����������
��5����xg��þ�Ͻ��ĩ�к���n molþ��zmol������24n+27z=x�٣�
�ٸ��ݷ�Ӧ��ϵʽ��Mg��MgO��Al��Al2O3�����õ���yg������ݴ���ʽΪ��40n+51z=y�ڣ�
���ݢ٢ڽ�ã�z=$\frac{3y-5x}{18}$mol����������Ϊ��27g/mol��$\frac{3y-5x}{18}$mol=$\frac{9y-15x}{2}$g���Ͻ���þ����������Ϊ��$\frac{\frac{9y-15x}{2}}{x}$=$\frac{17x-9y}{2x}$��
�ʴ�Ϊ��$\frac{17x-9y}{2x}$��
��6��װ�õ���װ˳�Ͻ����ᷴӦ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽���������������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���������˳��Ϊ����a���ӣ�e����d���ӣ�g����
�ʴ�Ϊ��edg��
��7����װ���е���a�������ǣ����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ʴ�Ϊ�����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
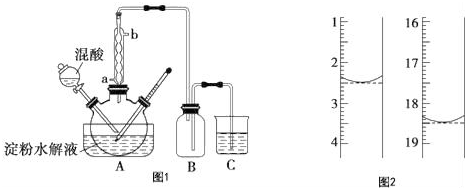
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ��������������ռ�������ζ�����Һ�������С����Ӧǰ�ζ��ܶ���Ϊ18.50mL����Ӧ��ζ��ܶ���Ϊ2.50mL�����Բⶨ���������Ϊ��18.50mL-2.50mL=16.00mL��
�ʴ�Ϊ��16.00��
������ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��
�ʴ�Ϊ������ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬Ҫ��ѧ��������ʵ�Ļ������ۺ�����֪ʶ�������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��
��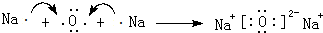 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ᾧ����ʱ�����д�����������ʱֹͣ���ȣ����������� | |
| B�� | �������ʱ��Ӧʹ�¶ȼ�ˮ�������Һ̬������� | |
| C�� | ��Һ����ʱ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ��� | |
| D�� | ��ȡ����ʱ����ȡ��Ҫ��ԭ�ܼ��������ܣ��Ҳ��������ʺ��ܼ���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ӦʽΪKClO4��s��+8e?�TKCl��s��+4O2?��l�� | |
| B�� | �ڸ��ȵ���У���Ϊ������������ԭ��Ӧ | |
| C�� | ��H���뷴Ӧ�ĸ������Խ�࣬��ֵԽС | |
| D�� | ����1 mol FeOת��8 mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ϩ�ļӳɣ����������ȡ����Ӧ�����Ƶ������� | |
| B�� | ����ʹ����ˮ��KMnO4��Һ�����Լ�����ϩ������ | |
| C�� | ��ͬ��������ϩ�ͼ�����ȫȼ�պ������ˮ��������ͬ | |
| D�� | ��ϩ�Ļ�ѧ���ʱ�����Ļ�ѧ���ʻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��C�����������ˮ����ĵ���ʽ
��C�����������ˮ����ĵ���ʽ ������ɫ�Ĺ���E�ĵ���ʽ
������ɫ�Ĺ���E�ĵ���ʽ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
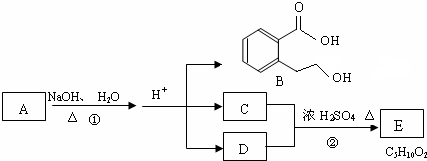
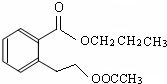 ��
��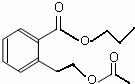 ��
�� ��
��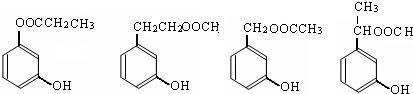 ��д������֮һ���ɣ���
��д������֮һ���ɣ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com