

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
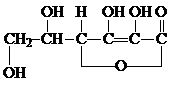 �������ʽΪ
�������ʽΪ
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
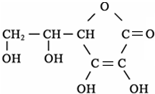 �������ʽΪ
�������ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
��ش��������⣺
��1��A��Ԫ�����ڱ��е�λ���� ��A��E�γɵĻ�����ĵ���ʽ�� ��
��2��C��ij���Ȼ����Ũ��Һ���Ը�ʴӡˢ��·���ϵĽ���ͭ���˷�Ӧ�����ӷ���ʽ�� ��
��3��B�ĵ�����D���⻯����һ�������·�Ӧ����BD����һ����Ļ�ѧ����ʽ�� ��
��4��F��������ˮ��Һ�����ԣ�ԭ���� �������ӷ���ʽ��ʾ����F�ĵ�����C��D�γɵ���Է�������Ϊ160�Ļ�������һ�������·�Ӧ�Ļ�ѧ����ʽ�� ��
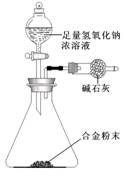
��5��A��F�γɵĺϽ�����Ҫ�Ĺ�ҵ���ϡ�ijͬѧ��ʹ����ƽ����ͼ��ʾ��װ�ã����ԲⶨijЩ���ݼ�������úϽ���AԪ�صĺ�������װ�����������������������Բ��ƣ�

��ʵ����Ҫ�ⶨ�������������Ͻ������m�Լ�a��b��
a�� ��
b�� ��
�ںϽ���AԪ�ص����������� ���ú�m��a��b��ʽ�ӱ�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ֣������������ѧ�ڵڶ����¿������ۣ���ѧ���� ���ͣ�ʵ����
A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�߶�1��ѧҵˮƽģ����ԣ��ģ���ѧ�Ծ��������棩 ���ͣ������
��1����3�֣��Ͼ�����»ᡱ�ѽ��뵹��ʱ���������ݺͳ��н�ͨ�����������ơ�
���������ݽ���������������ϡ����в��ϲ����ڹ����β��ϵ��� ������ĸ����
a��ʯ��ʯ b��ˮ�� c������
�ڹ����ͨ����������������ϡ����н���������������ʴ���� ������ĸ����
a�����Ͻ� b������ c����ͭ
�ۡ��ܽ����ܵ����ɾ۰����Ȳ��Ͻ��ɡ��۰��������� ������ĸ����
a���������� b�����ǽ������� c���л��߷��Ӳ���
��2����5�֣�����»ᡱ�ڼ䣬Ҫ�����˶�Ա��Ӫ���뽡����
�ټ�ʱ�����������˶�Աȡ������ɼ��Ļ�����֤���������Ӫ�����������������������ܵ������ࡢ �� ��
��ˮ�����߲˸���VC����֪VC�ĽṹΪ 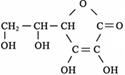 �������ʽΪ
�������ʽΪ
�����Ȼ�����Һ�м���VC��Һ����Һ�ɻ�ɫת��Ϊdz��ɫ��˵��VC���н�ǿ��____�ԡ�
�۷���Υ��ҩ�ﲻ���������������Ĺ�ƽ��������Ҳ�к��˶�Ա�����Ľ������ڰ�˹ƥ�֡���ù�ء�����ء�С�մ�ȳ���ҩ���У�����ѡ�ֲ��ɷ��õ��� ��
��3����7�֣�����ˮ���족����Ϊ�˶�Ա�ṩ����������������չʾ�Ŷ��Ͼ�����������
��PM2.5ָ�����ڴ����е�ֱ����2.5��m���ף��Ŀ��������PM2.5����ɻ���������Σ�����彡����ȼú���������ڿ���PM2.5�ĺ�����д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ ��
������β���к�����Ⱦ������NO��CO�������������ܼ�װ����ת����������ʹCO��NO��Ӧ����������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ ��װ�С���ת����������������ʹ����Ǧ���ͣ���ԭ���� ��
�ۺ���Ԫ�صķ�ˮ����������ꡣij��ȤС��̽��������Cr2O72������ˮ�Ĵ������������������ϣ������(NH4)2Fe(SO4)2��Cr2O72����ԭΪCr3+�����ð�ˮ��Cr3+ת������ܵ�Cr(OH)3���÷�����������ˮ���������е�������ԭ���� ��ָ����ʦָ���÷���������ķ�ˮ�����д��� �������ӷ��ţ����ܵ���ˮ�帻Ӫ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com