

���� ��1�������ж�����Ⱦ������ʯ����������������Ӧ������δ��Ӧ����������ƿ�з�����Ӧ�Ƕ�������ԭ�������̵������̣�
��2�����ݡ����̡�ʱ��������ʵĹ�ϵͼ�ж�ʱ�䣻��ͼ���֪����N2���ɿ�������ӦҺ��SO42-��Ũ���淴Ӧʱ��������࣬O2��H2SO3��Ӧ����H2S04��
��3��Mn��OH��2��ʼ����ʱpH=7.7�����м����Թ���NaHCO3��Һ��Ŀ��������̼���̣�Ҫ��ֹ�������̵����ɣ��ݴ��ж�pHֵ��MnSO4��NaHCO3��Ӧ����̼���̺������ơ�������̼��ˮ���ݴ˴��⣮
��� �⣺��1��ʯ����������������Ӧ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��ʯ�����ȥδ��Ӧ��������ֹ������Ⱦ��������ƿ�з�����Ӧ�Ƕ�������ԭ�������̵������̣���Ӧ����ʽΪMnO2+SO2=MnSO4��
�ʴ�Ϊ����ȥδ��Ӧ��������ֹ������Ⱦ������MnO2+SO2=MnSO4��
��2�����ݡ����̡�ʱ��������ʵĹ�ϵͼ��֪����Ӧ����ʱ����2.5��3Сʱ����ʱ�����ʽӽ�100%����ͼ���֪����N2���ɿ����������к���������Mn2+��O2��H2SO3��Ӧ����H2SO4�����Է�ӦҺ��SO42-��Ũ���淴Ӧʱ��������࣬
�ʴ�Ϊ��2.5��3Сʱ��Mn2+��O2��H2SO3��Ӧ����H2SO4��
��3��Mn��OH��2��ʼ����ʱpH=7.7�����м����Թ���NaHCO3��Һ��Ŀ��������̼���̣�Ҫ��ֹ�������̵����ɣ�����pHֵҪС��7.7��MnSO4��NaHCO3��Ӧ����̼���̺������ơ�������̼��ˮ����Ӧ�����ӷ���ʽΪMn2++2HCO3-=MnCO3+CO2��+H2O��
�ʴ�Ϊ��7.7��Mn2++2HCO3-=MnCO3+CO2��+H2O��
���� ������Ҫ�������Ʊ��ߴ�̼����ʵ�鷽����������������ͼ����ȷʶͼ���������ӷ�Ӧ�ı����ǽ��Ĺؼ�������ʵ���ۺ���ǿ���Ѷ��еȣ�


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | SO2��mol�� | O2��mol�� | N2��mol�� | Q��kJ�� |
| �� | 2 | 1 | 0 | Q1 |
| �� | 1 | 0.5 | 0 | Q2 |
| �� | 1 | 0.5 | 1 | Q3 |
| A�� | Q2=Q3��98.5kJ | |
| B�� | Q1=2Q2=2Q3=197kJ | |
| C�� | 2Q2=2Q3��Q1��197kJ | |
| D�� | �����������·�Ӧ����1molSO3�������98.5kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3��
������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3�� �dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ�
�dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ� ��]���״�Ϊԭ���Ʊ��������������֪��
��]���״�Ϊԭ���Ʊ��������������֪��| �۵�� | �е�� | ˮ���� | |
| �״� | -97.8 | 64.7 | ���� |
| ������ ��һԪ���ᣩ | 122.4 | 249.3 | ���£�0.17g 100�棺6.8g |
| ��������� | -12.3 | 198 | ���� |


 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | SiO2 | C | Na2O | K2O | Al2O3 | Fe2O3 |
| �������� | 59.20 | 38.80 | 0.25 | 0.50 | 0.64 | 0.16 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
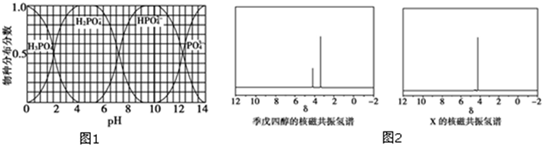
 ���뼾���Ĵ���
���뼾���Ĵ��� �������ʵ���֮��2��1 ��Ӧ
�������ʵ���֮��2��1 ��Ӧ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com