
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棬���������۵�Ϊ231�档װ��A�з�Ũ����B�з�MnO2�����������������������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ�����ش����и����⡣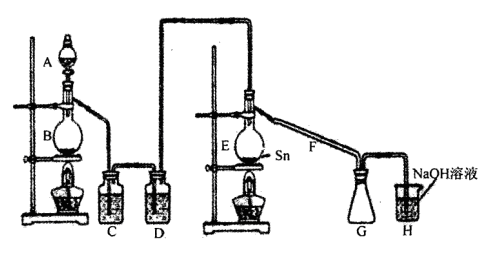
��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��
��
��1���õ��ܽ�A���Ͽں�B��������A���ɺ�ѹ��Һ©����1�֣���G��H֮�����Ӹ���װ�ã�1�֣�
��2��Cl2��H2O=Cl�D��ClO�D��H2O��2�֣�Sn��2Cl2 SnCl4��2�֣�
SnCl4��2�֣�
��3������ʳ��ˮ����ˮ����1�֣�Ũ���ᣨ1�֣�
��4����Һ©����1�֣�������ƿ��1�֣�������������2�֣�
��5��E��1�֣�231�棨1�֣�Sn���ۻ���1�֣�
��6��SnCl4��2H2O=SnO2��4HCl��2�֣�
��7�����ְ�ɫ������1�֣�
���������������1������װ�����������壬ѹǿ����Һ©���е������˳���ӵ�Բ����ƿ�У�Ӧ�õ��ܽ�A���Ͽ���B��������ƽ���Һ©����Բ����ƿ�е�ѹǿ�����Ȼ�������ˮ�⣬GΪ�ռ����Ȼ���װ�ã�Ӧ��G��H֮�����Ӹ���װ�÷�ֹH�е�ˮ��������Gװ�ã���Ϊ���õ��ܽ�A���Ͽ���B��������G��H֮�����Ӹ���װ�ã�
��2��HΪ����Ϊ��Ӧ���������������������Ʒ�Ӧ�����Ȼ��ơ��������ơ�ˮ����Ӧ���ӷ���ʽΪ��Cl2+2OH��=Cl��+ClO��+H2O����װ��ͼ��֪��E��Ϊ�������������������Ӧ������ˮ���Ȼ�������Ӧ����ʽΪ��Sn+2Cl2 SnCl4����Ϊ��Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2
SnCl4������Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2 SnCl4��
SnCl4��
��3�����Ȼ�������ˮ�⣬����װ��E������Ӧ���������װ��C������Ϊ���ջӷ�����HCl���ñ���ʳ��ˮ����HCl��װ��D������Ϊ����ˮ�������������壬��Ũ�������ո����Ϊ������ʳ��ˮ��Ũ���
��4������AΪ��Һ©����BΪ������ƿ��F���������������Ȼ�����������Ϊ����Һ©����������ƿ��������������
��5�����ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ�������Ӧ�����E�ƾ��ƣ������¶�Ӧ���ڽ��������۵㣬�����ڻ���ֹͣ���ȣ���Ϊ��E��231�����ڻ���
��6�����Ȼ�����ˮǿ��ˮ�⣬��ˮ��ԭ����֪��Ӧ����Sn(OH)4��HCl������֮һ�ǹ�̬����������˵��Sn(OH)4�ֽ�����SnO2��H2O�������Ȼ���ˮ������SnO2��HCl����Ӧ����ʽΪ��SnCl4+2H2O=SnO2+4HCl����Ϊ��SnCl4+2H2O=SnO2+4HCl��
��7�����Ȼ�����ˮǿ��ˮ������SnO2��HCl��SnO2�ǹ��������HCl��Ͽ����е�ˮ���������ְ�ɫ��������Ϊ�����ְ�ɫ������
���㣺������ʵ�����Ʒ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��һ֧�Թ��з���һ���С��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ������ܿڴ�����һ��պ��Na2CO3��Һ���������Թܼ��ȣ��۲�����Ӧһ��ʱ���ֹͣ���ȡ��ش��������⣺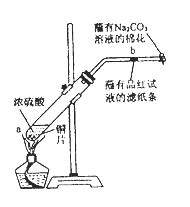
��1��д��a����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3�����Թ��е�Һ����ȴ���Թ��ϲ�Һ�嵹ȥ���ٽ�ʣ��������������ˮ�У��ɹ۲���Һ�� ɫ��
��4���������ܿڴ�����һ��պ��Na2CO3��Һ��������������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��5������Ũ��Ϊ18 mol/L��Ũ����100 mL�������ͭƬ������ʹ֮��Ӧ����ԭ�����ᣨѡ����ڡ��������ڡ���С�ڡ��� 0��9 mol ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��������װ�ó�ȡ�ռ����������������о������ʡ�������������⡣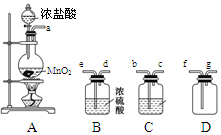
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��2��������������������ӿڵ�����˳��Ϊa��___________________��g��
��3��װ��B��Ũ�����������____________________________________________________________��װ��C���Լ������___________________________________��
��4��ijͬѧ��Ϊ��������ȱ��β������װ�ã���������ķ����л�����װ�ò�ע���Լ���
| |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ClO2��Ϊ�����������������ж��������������й㷺��Ӧ��ǰ����ijͬѧ����ͼ��ʾ��װ���Ʊ�ClO2���壬��Ӧԭ��Ϊ���Ͳ�����Һ��KClO3��ĩ��60��ʱ��Ӧ�Ƶ�ClO2���¶ȹ�����Ͷ���Ӱ������Ч�ʣ�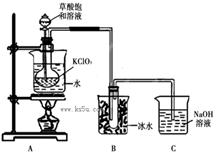
��֪��Ϣ����ClO2��һ�ֻ���ɫ�д̼�����ζ�����壬�۵�-59�棬�е�11.0�档��Ӧ���������ơ�
�ڲ���������ǿ�ڴ���Ķ�Ԫ���ᣬ��Ӧ�ĸ��Σ�CaC2O4�������ڴ��ᣬ������ǿ�ᣬ������һ�ֻ�ԭ�Խ�ǿ�����ʡ�
��1���Ʊ�ClO2�Ļ�ѧ����ʽ��2KClO3+H2C2O4= 2KHCO3+2ClO2��������˵����ȷ����
| A��KClO3�ڷ�Ӧ�еõ����� |
| B��ClO2���������� |
| C��H2C2O4�ڷ�Ӧ��ʧȥ���� |
| D��1mol KClO3�μӷ�Ӧ��2mol����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��֤ʵľ̿��ŨH2SO4�ķ�Ӧ��������������̽�����֤(��ͷ��ʾ���������)��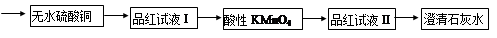
�ش��������⣺
��������������ʲô����֤��������H2O��CO2��SO2��
�� �� ��
(2)����KMnO4��Һ�������� ���ܷ�����ˮ�������Ը��������Һ? ��(��ܡ����ܡ�)����д����ѧ��Ӧ����ʽ �����ش��ܣ���˿ղ��ô��⣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ�ִ�����Ⱦ��о���NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壬ij��ѧʵ�鰮��С����̽��SO2�����ʣ�������·�����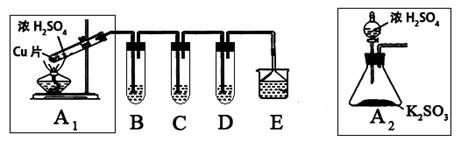
��1��B��C��D�ֱ����ڼ���SO2�Ļ�ԭ�ԡ������Ժ�Ư���ԡ�����B��C�ֱ�Ϊ��ˮ�������ˮ��Һ����D����ʢ�Լ�Ϊ_________��B�з�Ӧ�����ӷ���ʽΪ��_________________��
��2��Ϊ��ʵ����ɫʵ���Ŀ�꣬ijͬѧ���������������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��ǣ�________________________________����дһ�㼴�ɣ���
��3��E���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ���֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2mol/L���ᡢ1mol/L BaCl2��Һ��1mol/L Ba(OH)2��Һ��Ʒ����Һ������ˮ��
�����ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32���� SO42���� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ___________________________________________________ | _________________________ ______________________________________________ |
| ����3��_______ _______________________ ___________________________________________________ | _________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��ѧϰ����������ʱ������������ͭ�ķ�Ӧ��������о�����������и��⡣
��1���ڼס��������ձ��У��ֱ�װ��40mLŨ�Ⱦ�Ϊ2mol��L��1��ϡ�����ϡ���ᣬ�������и����� 4g��״ͭ˿���۲��������������ʵ�鱨�棺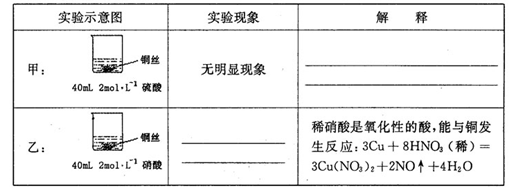
��2����ַ�Ӧ���ס����ձ���ϣ���ʹ֮��ַ�Ӧ������������Һ����Ϊ____ ��ʣ�����������Ϊ g
��3��������������Һ���V��V>40mL���ɱ䣬�������ݲ��䣬��
�ٵ��ס����ձ���ϳ�ַ�Ӧ����Һ��ֻ��һ������ʱ��V=____ mL����Ҫ����Һ�е�Cu2+������ȫ��Ӧ��NaOHʹ��Һ��pH����Ϊ____ ����֪KsP[Cu��OH��2]=2.2��l0��20,1g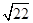 =0.7��
=0.7��
���ܷ�ͨ��������Һ����ĸı䣬ʹͭ˿�ڼס����ձ���ϳ�ַ�Ӧ����ȫ�ܽ�? ��д����������________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���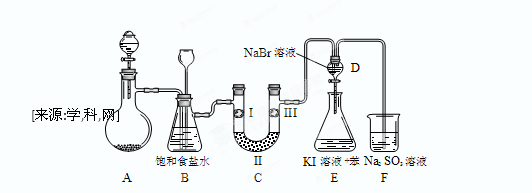
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾����塾��Ҫ�ɷ�ΪCa��ClO��2����Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ�� ��
��2��װ��B�б���ʳ��ˮ�������� ��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
| | a | b | c | d |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
X��Y��Z���ֲ�ͬ��������ͼ��ʾ��ת����ϵ����X�������� (����)��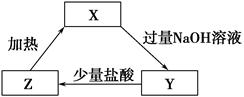
| A��Al2O3 | B��SiO2 | C��CO2 | D��NH4Cl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com