

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O2��Na2O | B��Na��NaOH | C��Na2O2��Na | D��Na��Na2O |
�鿴�𰸺ͽ���>>
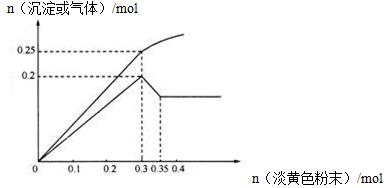
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
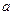 g������Ͷ�뵽
g������Ͷ�뵽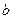 gˮ���������С�
gˮ���������С� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���٢� | C���ڢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O | B��CO2 | C��Ca��OH��2 | D��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �֣���ʯ��ʯ��ˮ������Ϊԭ�ϣ������ȡ�ռд���йط�Ӧ�Ļ�ѧ����ʽ����˵��������Ӧ���͡�
�֣���ʯ��ʯ��ˮ������Ϊԭ�ϣ������ȡ�ռд���йط�Ӧ�Ļ�ѧ����ʽ����˵��������Ӧ���͡��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �����Ʋ⣬ʵ�����б������������Ƶ���ȷ�����ǣ� ��
�����Ʋ⣬ʵ�����б������������Ƶ���ȷ�����ǣ� ��| A��������ˮ�� | B�������ڹ��ƿ�� |
| C���������������ܷ��ڹ��ƿ�� | D��������ú���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������� | B�������� | C�������� | D���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com