
| A����ȥʯӢ�л��е��������ۣ��ɼ�������������Һ�ܽ����� |
| B����ȥ���������л��е��������ᣬ���뱥������������Һ���Һ |
| C����֪��Ksp��CuS��<Ksp��FeS�����ɼ�����������ܵ����FeS��ʹˮ��������Cu2+ת���������������ȥ |
| D������������FeBr2��FeCl2��Һ�У�����������ˮ���ټ�CCl4��ȡ��Һ���Գ�ȥFeCl2��Һ�е�FeBr2 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����NH3��H2O��Һ����Al3+��Mg2+��Ag+ |
| B����Ba��NO3��2��Һ����Cl����SO2��4��CO2��3 |
| C���ú˴Ź���������1-������2-����� |
| D��������������ͭ����Һ�����ȩ�ͼ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ᣨ��ȩ�����������Ƶ�������ͭ������ |
| B���������ӣ���������ˮ������ |
| C�������飨�嵥�ʣ�������������������Һϴ�ӣ���Һ |
| D���������������ᣩ�����뱥��̼������Һϴ�ӣ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ױ����ұ� | B���������������顢�ȱ� |
| C���Ȼ��ơ��Ȼ��ء��Ȼ�ͭ | D��̼�����ơ��Ȼ��ơ��Ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ũ��ˮ��ȥ���еı��� |
| B��������Cu(OH)2����Һ���𱽡���ȩ������ |
| C���ڼ��������£����Ҵ���ȥ���������е����� |
| D�����������NaOH��Һ��ϼ��Ⱥ��ټ�����������Һ��������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
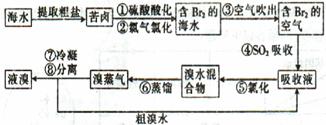
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

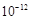
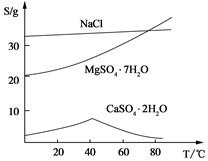
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2mol��L��1HCl��Һ��������������ͨ�� | ���� ���ۣ� |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ� | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ� |
| ����4����B�Թܣ� | ��������ɫ������ ���ۣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢ� | B���٢ۢݢڢ� | C���ڢݢ٢ۢ� | D���ڢ٢ۢݢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com