
��1������ʵ��������ʵ����ʵ����������ȷ���� ������ţ���
| A����������ƽ����17.55g�Ȼ��ƾ��� |
| B��̼������Һ�����ڴ����������Լ�ƿ |
| C���ø����pH��ֽ�ⶨ������ˮ��pH |
| D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ�� |


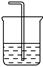
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | | | Ũ��ˮ |
| Բ����ƿ���Լ� | | KMnO4 | |
��1��ABCE��4�� ÿ��ѡ1������©1������1�֣�
��2���٣���4�֣�
���� ����ʳ��ˮ���� O2 Cl2 NH3 ��Һ©�����Լ� H2O2����H2O�� Ũ���� Ũ��ˮ Բ����ƿ���Լ� MnO2����Na2O2�� KMnO4 NaOH����CaO��
��Cl2��2OH��=ClO����Cl����H2O ������������ˮ��ϡ���ᣩ����������
CCl4(�����Ȼ�̼)
���������������1��A����������ƽ����ֻ�ܾ�ȷ��0.1g������B��̼������Һ�ʼ��Ի�Ͳ������еĶ������跴Ӧ������ճ�Ե����ʹ����ƣ���ʹ�������Ͳ���ƿճ��һ�𣬴���C���ø����pH��ֽ�ⶨ������ˮ��pH��������ˮ��Ư���Ի�ʹ��ֽ������ɫ���۲죬����D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ����ȷ��E������е�����ˮ���������뱥��FeCl3��Һֱ�����ɺ��ɫҺ�壬������ȡFe(OH)3���� �ij��÷���������F����ȥCO2�����л��е�����HCl�����Խ�����ͨ�뱥��̼��������Һ����ȷ����ѡΪABCE����2������ͼ�ֱ����Ʊ����������ԭ��
��Ҫ��Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��_�ҹܿڵ������������ܰ�Һ���ų����ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ����ʳ��ˮ����һ�����ȥ�Ȼ��⣬��һ�����������ܽ��ԡ����������������Ƶķ�Ӧ���ӷ���ʽΪCl2��2OH��=ClO����Cl����H2O��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� ������������ˮ��ϡ���ᣩ�����������������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���ΪCCl4(�����Ȼ�̼)�����ʵ��ܶȱ�ˮ���������ܽ������С����� O2 Cl2 NH3 ��Һ©�����Լ� H2O2����H2O�� Ũ���� Ũ��ˮ Բ����ƿ���Լ� MnO2����Na2O2�� KMnO4 NaOH����CaO��
���㣺���鳣��ʵ�����������ʵ�����Ʊ�����Ļ���ԭ����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���÷�ӦI2(s)+Cl2(g)=2ICl(l)��ʵ���ҿ�������ͼ��ʾװ�ã����ȡ��г���������ȥ����ȡ����IC1��
��֪��ICl���۵�Ϊ13.9�棬�е�Ϊ97.4�棬��ˮ�⣬���ܷ�����Ӧ��
ICl(l)+Cl2(g)=2ICl3(l)
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��____________��
��2��װ��B��������______��������װ��F����װ��E��������____________��
��3�����Ƶõ�ICl����������ICl3���ʣ��ᴿ�ķ�����______ (���ţ���
| A������ | B�������ᾧ | C������ | D����Һ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֡�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ�����������ϸߵ������ӣ�Ȼ������ת��Ϊ��ͨ�����ӣ�����������Ժ��ų�����ʵ�����õ�������������ͼ��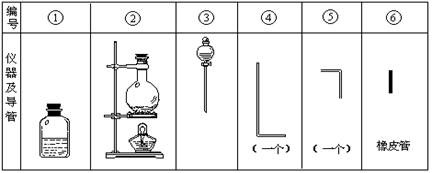
����Ҫ����д���пհף�
��1����װ��������װ��ʱ��Ӧѡ�õ�����������Ϊ (��дͼ�б��)��
��2����ʵ�����ʱ����������������ҵ�˳�����������ĸ����������ܵı������Ϊ ��
��3�������ٵ���Ƥ��������Ӧ��2����ԭ���� ��
��4����ʵ��������10mol·L��1��NaOH��Һ500mL���õ�����������������ƽ���ձ��⣬�����õ��������� (����������) ������ʱ������ͼ����������ҺŨ�� (�ƫ�ߡ���ƫ�͡�)��
��5��ʵ��ʱ��������ClO����H2O2��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�������������������������������ʡ�����ʵ���������£�
��ش��������⣺
��1������1���õ��IJ���������������____________��
��2������Һ3��ȡ�������������ӷ���ʽΪ ��
��3������д����֤��Һ1����NH4+��ʵ����̣�_______________________________________________��
��4��ʵ������Fe2O3��CO��Ӧ����ȡ����Fe��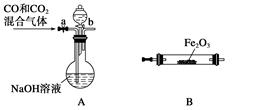

���밴���������ҵķ����������и�װ�ã�˳��ΪA�� ��
�ڼ��װ��A�����Եķ����� ��
���ڵ�ȼB���ľƾ���ǰ��Ӧ���еIJ�����_______________________________________����װ��C��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
(1)����ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��_________ _������֮�⣬װ���е�һ�����Դ����� ��
(2)Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������________ ___��
(3)������60mL 0.25mol��L-1 H2SO4��50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ������ʵ����ȣ����ų������� �����ȡ���������ȡ�������ʵ���������ȷ���������к��� ���ȡ�������ȡ���
(4)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز�������������
B����������������
C��һ��Ѹ�ٵ���
(5)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ���
B���ҿ�ӲֽƬ�ò���������
C����������ձ�
D���������¶ȼ��ϵĻ��β���������ؽ���
(6)ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ���̲��ŷḻ����Դ����ˮ�ۺ����õ�����ͼ���¡�
��1����NaCl��ԭ�Ͽ��Եõ����ֲ�Ʒ��
�� ��ҵ����NaCl�Ʊ������ƵĻ�ѧ����ʽ��_______________________________��
�ڵ���Ȼ���ϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������Һ��ȫ���գ�����������Һ����һ�����ʣ�д����Ӧ�Ļ�ѧ����ʽ��____________________________��
��2����������κ��±ˮ���̺��ŷḻ��þ��Դ����������;���ɻ�ý���þ��
±ˮ Mg��OH��2
Mg��OH��2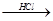 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2 Mg
Mg
���У���MgCl2��6H2O��ȡ��ˮMgCl2�IJ���װ�ã�����̨���ƾ������ԣ����£�
����ͼ�У�װ��a�� �� ��˫�����͵�����ɡ�
��ѭ�����ʼ������� ��
����ȡ��ˮ�Ȼ�þ�������Ȼ�����ڵ������½��У�ԭ���� ��
��װ��b���������ʿ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijNa2CO3��Ʒ�л���һ������Na2SO4 (��������ᾧˮ����ij��ѧ��ȤС��������ַ����ⶨ����Ʒ��Na2CO3�������������Իش��������⡣
����һ���������з������Na2CO3���������Ĝy��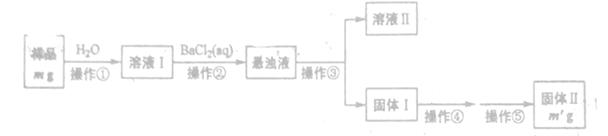
(1)�����ۺܵ͢����Ʒֱ�Ϊ_______��
(2)���������١����У�ʹ�õ�����������______(��������)��
(3)�жϲ����ڷ���ɵķ�����______
��������������ͼʵ��װ�ã��г�������ʡ�ԣ�.ѡ�������Լ�: a.Ũ����b.����NaHCO3��ҺC.6mol/L����D.2mol/L����, e.��ʯ��f. ��ˮCaCl2,�y����Ʒ��Na2CO3,������������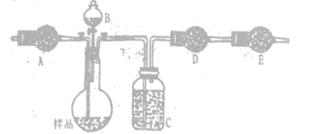
(4)��д���пո�
| ���� | �Լ� | ������Լ���Ŀ�� |
| A | | �������ʱϴȥCO2 |
| B | | ʹ��Ʒ��ַ�Ӧ�ų����� |
| C | a | |
| D | e | �������CO2 |
| E | e | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ�r������ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������Ϊ���ijʳƷ���������κ�����ͨ����1kg��Ʒ�к�NaNO2��������)��ij�о�С���������������ʵ�鷽����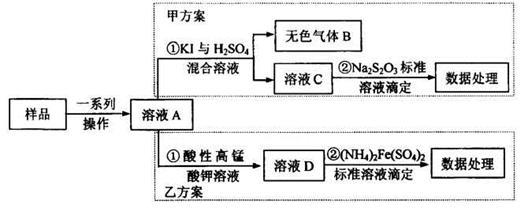
(1)��ɫ����B�������Ժ���ɫ��B�Ļ�ѧʽΪ_______д���������з�Ӧ�����ӷ���ʽ_______
(2)��ɲ���ƽ�ҷ������з�Ӧ�����ӷ���ʽ
MnO4-+ NO2-+ = Mn2++ NO3-+ ,
(3)�ҷ�������������100mL0.0010mol/L(NH4)2Fe(SO4)2����Һ������ȷ������Ʒ����������Ҫ�������У���Ͳ���ձ���_______������Һʱ�����ݵIJ���������______
(4)��ȡ��Ʒag�����ҷ������вⶨ��ȷ��ȡ12.00mL0.0005mol/L�����Ը��������Һ����ͯ������ҺA��Ӧ����Ӧ����Һ��0.0010mol/L(NH4)2Fe(SO4)2����Һ�ζ�����ɫ��Һ�պ���ȥ���ظ�����ʵ��2�Σ�ƽ������(NH4)2Fe(SO4)2��Һ10.00mL.��1kg��Ʒ��NaNO2������Ϊ_______mg.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com