
�����й�ʵ���������ȷ����
A����ʪ���pH��ֽ�ⶨ��Һ��pH���ⶨ���ƫС������Һһ��������
B���к͵ζ�ʵ���У���ƿ������ˮϴ����ʹ�ã��ζ���������ˮϴ��������� ���ô�װҺ��ϴ��ʹ��
C������ˮ�����Һ©�������������Ҵ������ã��ܽ�����ȡ���Ҵ���
D������FeCl2���ʵ�FeCl3��Һ��ͨ������C12��ּ������ɣ��ɵõ�������FeCl3����
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
M��N����Һ�ֱ�������ʮ���������е����ֺ��������ӣ�K����Na����H����
NH4����Fe3���� A13+��Cl-��OH-��NO3-��S2-��CO32-��SO42-����֪����Һ�������Ӹ�����ͬ��M��Һ���������ֻ�����֣���N��Һ���������Ӧ����( )
A��OH-��CO32-��SO42- B��S2-��Cl-��SO42-
C��CO32-��NO3-��S2- D��Cl-��SO42-��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʹˮ�ĵ���ƽ�����ƣ���ˮ��Һ�����Ե�����
A��Al3���������� B��OH�� C��H�� D��HCO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PH��2��A��B��������Һ��1ml���ֱ��ˮϡ�͵�1000ml������Һ��PH����Һ����zV�{�Ĺ�ϵ����ͼ��ʾ��������˵����ȷ����
A��A��B��������Һ�����ʵ���Ũ��һ�����
B��ϡ�ͺ�A����Һ�����Ա�B����Һ��ǿ
C��a��5 ʱ��A�����ᣬB��ǿ��
D����A��B�������ᣬ��5��a ��2
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l)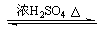 CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��
��1�����о�С���ʵ��Ŀ����__________________��
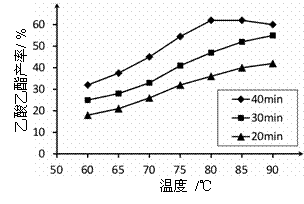 ��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����___________���ߣ��С�ڡ��������ڡ����ڡ�����
��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����___________���ߣ��С�ڡ��������ڡ����ڡ�����
��3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������______��д����������
��4��ij�¶��£���0.10 mol CH3COOH����ˮ���1 L��Һ��
��ʵ�����ѵ���Ĵ������ռԭ�д������������1.3%������¶���CH3COOH�ĵ���ƽ�ⳣ��K=____________________����ˮ�ĵ�����Բ��ƣ��������Դ������Ũ�ȵ�Ӱ����Բ��ƣ�
�������Һ���ټ���__________mol CH3COONa��ʹ��Һ��pHԼΪ4������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
(1)������ȼ������ʱ������N2��O2�ķ�Ӧ�� ���ǵ�������β���к���NO��ԭ��֮һ��
���ǵ�������β���к���NO��ԭ��֮һ��
����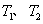 �¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�Ӧ
�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�Ӧ __________0(�>����<��)��
__________0(�>����<��)��
���� �¶��£���2L�ܱ������г���10molN2��5mo1O2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ������
�¶��£���2L�ܱ������г���10molN2��5mo1O2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ������ ___________________�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1 mol����ﵽƽ���N2��ת����Ϊ____________��
___________________�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1 mol����ﵽƽ���N2��ת����Ϊ____________��
 (2)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2���������ų�����Һ������NO2��
(2)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2���������ų�����Һ������NO2��
�������ĵ缫��ӦʽΪ_____________________��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ�� ���ɡ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_______________��
���ɡ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_______________��
(3)һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol��L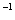 �Ĵ�����b mol
�Ĵ�����b mol L
L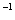
 ��Һ�������ϣ���ַ�Ӧ����Һ�д���
��Һ�������ϣ���ַ�Ӧ����Һ�д���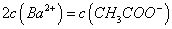 ����û����Һ�д���ĵ��볣��Ka=______________________(�ú�a��b�Ĵ���ʽ��ʾ)��
����û����Һ�д���ĵ��볣��Ka=______________________(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��B��Ԫ��ԭ������Ϊx����ôԭ������Ϊx+1��Ԫ��λ��
A����A�� B����B�� C����B�� D����A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ӳ�����ͬ������Ԫ��X��Y��Z����������������Ӧˮ�����������ǿ����˳��
Ϊ��HXO4��H2YO4��H3ZO4�������жϴ������
A.ԭ�Ӱ뾶 X��Y��Z B.��̬�⻯���ȶ���X��Y��Z
C.Ԫ��ԭ�ӵõ�������X��Y��Z D.������������Ӧ����X��Y��Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ܻ�óɹ����� �� ��
A���Ҵ���2mol��L��1��������Һ��ϼ��ȵ�170������ϩ
B��������ˮ��Ӧ��ȡ�屽
C��Ҫ����ϩ���Ƿ���������ױ�����ǡ����ʵ�鷽�����ȼ����������Ը��������Һ��Ȼ���ټ�����ˮ
D����ʯ�뱥��ʳ��ˮ��������Ȳ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com