
�ο��������ʵ��۵㣬�ش��й����⡣
| ���� | NaF | NaCl | NaBr | NaI | NaCl | KCl | RbCl | CsCl |
| �۵�/�� | 995 | 801 | 755 | 651 | 801 | 776 | 715 | 646 |
| ���� | SiF4 | SiCl4 | SiBr4 | SiI4 | SiCl4 | GeCl4 | SnCl4 | PbCl4 |
| �۵�/�� | ��90.2 | ��70.4 | 5.2 | 120.0 | ��70.4 | ��49.5 | ��36.2 | ��15.0 |
(1)�Ƶ�±���P��������Ȼ�����۵���±���Ӽ���������ӵ�__________�йأ�����__________�������۵����ν��͡�
(2)���±���P�衢�ࡢ����Ǧ���Ȼ�����۵���________________�йأ�����________________����________________�����۵��������ߡ�
(3)�Ƶ�±������۵����Ӧ�Ĺ��±������۵�ߵö࣬����__________�йأ���Ϊһ��__________��__________���۵�ߡ�
(1)�뾶���뾶��(2)��Է�����������Է������������Ӽ���������(3)�������͡����Ӿ��塡���Ӿ���
������(1)���Ӿ�����ۡ��е���������Ӽ���ǿ�������Ӽ���ǿ����ȡ�������Ӱ뾶�����������ĵ������ͨ��rԽС���������Խ�࣬�����Ӽ�Խǿ���ۡ��е�Խ�ߡ�
(2)��Ŀ�г��ֵ�Ϊ���Ӿ��壬��Ϊ��ɺͽṹ���Ƶķ��Ӿ��壬���ۡ��е�ߵ�ȡ���ڷ��Ӽ��������������Ӽ���������ȡ������Է��������Ĵ�С����Է�������Խ���ۡ��е�Խ�ߡ�
(3)һ���ۡ��е�ߵͣ�ԭ�Ӿ���>���Ӿ���>���Ӿ��塣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�����У������֮��Ľ������(����)
A�������� B�����ۼ�
C�����Ӽ��� D�����Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������У���̬ʱֻ���γ����Ӿ������(����)
A���ǽ��������� B���ǽ�������
C��ǿ�� D��ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������γ�����Ϊ�����д���(����)
A�����������Ӽ�������
B������ԭ�Ӽ�������
C�����������������ɵ��Ӽ�������
D������ԭ�������ɵ��Ӽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ���̼��K3C60��ʵ���֪�������������Ӿ��壬�������õij����ԡ����й���K3C60����ɺͽṹ�ķ�����ȷ����(����)
A��K3C60�м������Ӽ������м��Լ�
B��1 mol K3C60�к��е�������ĿΪ63��6.02��1023
C���þ���������״̬���ܵ���
D�������ʵĻ�ѧʽ��дΪKC20
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������У����۵��ɸߵ��͵�˳��������ȷ����(����)
A��CH4��SiH4��GeH4��SnH4
B��KCl��NaCl��MgCl2��MgO
C��Rb��K��Na��Li
D�����ʯ��SiO2>NaCl>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E<F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�������ɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ�������
�����������Ϣ���ش���������(����ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ)��
(1)A��B��C��D�ĵ�һ��������С�����˳��Ϊ______________________��
(2)B���Ȼ�����۵��D���Ȼ�����۵�________(��ߡ��͡�)��������
________________________________________________________________________
________________________________________________________________________��
(3)F�ĺ�������Ų�ʽ��______________________________��F�ĸ�������A�ļ��⻯���γɵ������ӵĻ�ѧʽΪ__________��
(4)A��F�γ�ij�ֻ�����ľ����ṹ����ͼ��ʾ(����A�ԣ�3��)�����仯ѧʽΪ
____________��(ÿ�������ʾ1��ԭ��)
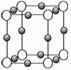
(5)A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ����仯ѧʽΪ____________________���侧���������Ļ�ѧ������Ϊ________________���侧��������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ����ӵĿռ乹���������ζ�����ƽ���������Σ����й������Ȼ����ӿռ乹�͵�����������ȷ����(����)
A��PCl3������P����sp3�ӻ�
B��PCl3������P��Cl�����ڼ��Թ��ۼ�
C��PCl3�������������ۼ����ܡ����Ǿ����
D��PCl3�ǷǼ��Է���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com