
 Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��| m |
| M |
| n |
| V |
| n |
| V |
| 27g |
| 180g/mol |
| 0.15mol |
| 0.5L |
| n |
| V |


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��
ʵ����Ʒ�������Ǿ���(Ħ��������180 g/mol)������ˮ���ձ�������ƿ(500 mL)��ҩ�ס���ͷ�ιܡ���Ͳ��
(1)��ȱ�ٵ�������_ ��
(2)���ж�����ƿ����ʹ�÷�������������ȷ����_ _��
A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
B������ƿ������ˮϴ�������ñ�������ע��Һ��ϴ
C��������Һʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
D������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
(3)ʵ������ȡ�þ��������Ϊ__ __�����ʵ���Ũ��_ _��
(4)����0.1 mol/L��NaCl��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ���__ ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���Ӷ�ȡ�̶�
C��ԭ����ƿϴ����δ���� D������ʱҺ�泬���˿̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����һ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��
| ������ע��Һ |
| ���250mL(�ܶȣ�1.08g·mL-1) �������ţ�1003203 2 ��Ч�ڣ���2013��10�� 5% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ̫ԭ���и���10�·��¿���ѧ�Ծ� ���ͣ�ʵ����
(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��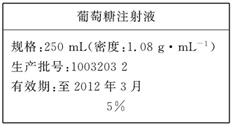
ʵ����Ʒ�������Ǿ���(Ħ��������180 g/mol)������ˮ���ձ�������ƿ(500 mL)��ҩ�ס���ͷ�ιܡ���Ͳ��
(1)��ȱ�ٵ�������_ ��
(2)���ж�����ƿ����ʹ�÷�������������ȷ����_ _��
A��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
B������ƿ������ˮϴ�������ñ�������ע��Һ��ϴ
C��������Һ ʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
ʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
D������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
(3)ʵ������ȡ�þ��������Ϊ__ __�����ʵ���Ũ��_ _��
(4)����0.1 mol/L��NaCl��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ���__ ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���Ӷ�ȡ�̶�
C��ԭ����ƿϴ�� ��δ���� D������ʱҺ�泬���˿̶���
��δ���� D������ʱҺ�泬���˿̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��
|
������ע��Һ |
|
���250mL(�ܶȣ�1.08g��mL-1) �������ţ�1003203 2 ��Ч�ڣ���2013��10�� 5% |
ʵ����Ʒ�������ǹ��塢����ˮ���ձ�������ƿ(500 mL)��ҩ�ס���ͷ�ιܡ���Ͳ��
(1)��ȱ�ٵ������� ��
(2)���ж�����ƿ��ʹ�õ������в���ȷ���� ��
A������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
B��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
C������ƿ������ˮϴ��������5%������ע��Һϴ
D��������Һʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
(3)ʵ������ȡ�ù��������Ϊ g����������ע��Һ�����ʵ���Ũ�� mol/L.��
(4)���Ƹ�������ע��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ��� ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���ӿ̶���
C������ƿϴ����δ���� D������ʱҺ�泬���˿̶���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com