
ij��Ԫ�ᣨH2A������ʽ�������룺H2A=H++HA����HA��![]() H++A2������������������Һ��˵����ȷ����
H++A2������������������Һ��˵����ȷ����
��0.01mol/L��H2A��Һ����0.01mol/L��NaHA��Һ����0.02mol/L��HCl��Һ��0.04mol/L��NaHA��Һ�������ϣ���0.02mol/L��NaOH��Һ��0.02mol/L��NaHA��Һ�������ϣ�
A��������Һ��c(HA��)Ũ�ȴ�С���ۣ��ڣ��٣���
B����Һ����һ��������OH��
C����Һ���д���ˮ��ƽ�⣺HA��+H2O![]() H2A+OH��
H2A+OH��
D����Һ�����й�����Ũ�ȹ�ϵ��c(HA��)+c(A2��)=c(Na+)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ����3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A��H++HA����HA�� H++A2������������������Һ��
H++A2������������������Һ��
�� 0.01mol?L1��H2A��Һ
�� 0.01mol?L1��NaHA��Һ
�� 0.02mol?L1��HCl��Һ��0.04mol?L1��NaHA��Һ��������
�� 0.02mol?L1��NaOH��Һ��0.02mol?L1��NaHA��Һ��������
���й�������������Һ��˵����ȷ���ǣ� ��
A����Һ���д���ˮ��ƽ�⣺HA�D+H2O  H2A+OH�D
H2A+OH�D
B����Һ�����У�c(HA��)+2c(A2��)=c(Na+)
C����Һ�����У�c(OH��)=c(H+)+c(HA��)
D��������Һ��c (HA��)Ũ�ȴ�С���ۣ��٣��ڣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ѡ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A��H++HA-��HA- H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ��������
H++A2-����������������Һ��0��0lmol/L��H2A��Һ��0��0lmol/L��NaHA��Һ
��0��02mol/L��HC1��Һ��0��04mol/L��NaHA��Һ�������Ϣ�0��02mol/L��NaOH��Һ��0��02mol/L��NaHA��Һ��������
���й�������������Һ��˵����ȷ���� �� ��
A����Һ���д���ˮ��ƽ�⣺HA-+H2O H2A+OH-
H2A+OH-
B����Һ�����У�c��HA-��+c��A2һ��=c��Na+��
C����Һ�����У�c��OHһ��=c��H+�� +c��HA-�� +2c��H2A��
D��������Һ��c��HA-��Ũ�ȴ�С����>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A=H++HA����HA�� H++A2������������������Һ��˵����ȷ����
H++A2������������������Һ��˵����ȷ����
��0.01mol/L��H2A��Һ����0.01mol/L��NaHA��Һ����0.02mol/L��HCl��Һ��0.04mol/L��NaHA��Һ�������ϣ���0.02mol/L��NaOH��Һ��0.02mol/L��NaHA��Һ�������ϣ�
A��������Һ��c(HA��)Ũ�ȴ�С���ۣ��ڣ��٣���
B����Һ����һ��������OH��
C����Һ���д���ˮ��ƽ�⣺HA��+H2O H2A+OH��
H2A+OH��
D����Һ�����й�����Ũ�ȹ�ϵ��c(HA��)+c(A2��)=c(Na+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ�㶫����У�߶��ڶ�ѧ������������ѧ���� ���ͣ�ѡ����
ij��Ԫ�ᣨH2A������ʽ�������룺H2A ��
H++HA����HA��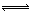 H++A2������������������Һ��˵����ȷ����
( )
H++A2������������������Һ��˵����ȷ����
( )
��0.01mol/L��H2A��Һ ��0.01mol/L��NaHA��Һ
��0.02mol/L��HCl��Һ��0.04mol/L��NaHA��Һ��������
��0.02mol/L��NaOH��Һ��0.02mol/L��NaHA��Һ��������
A����Һ��������Ũ�ȴ�С˳���ǣ�c(H2A)��c(H��)��c(HA��)��c(A2��)��c(OH��)
B����Һ�����й�����Ũ�ȹ�ϵ��c(HA��)��2c(A2��)��c(H2A)��c(Na+)
C��������Һ��c(HA��)Ũ�ȴ�С���ۣ��٣��ڣ���
D����Һ�����й�����Ũ�ȹ�ϵ��c(HA��)��c(A2��)��c(OH��)=c(Na+)��c(H��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com