
ij����С��Ϊ�˼�������ˮ��Ӧ�IJ���������ͼװ��(�г�װ��ʡ��)��������U�ι��ڼ�������ú�ͺͼ����ƿ飬�ٴ�U�ιܸ߶˼���ˮ(���з�̪)���ϳ�������һ�������ͭ˿��
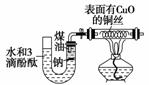
���ݷ�Ӧ�й۲쵽�����ش��������⣺
(1)�����Ƶı仯����______________________________��
(2)U�ι�����Һ����ɫ ____________________��
˵����________���ɡ�
(3)ͭ˿�ı仯���� ______________________________��
˵����________���ɣ���ȥ�������װ�ã�������������ķ���_____________________________________________________________��
(4)��a g����b mLˮ��ȫ��Ӧ�������Һ�����ʵ�����������________________��
��������������װ��������ˮ��Ӧʵ��ĸĽ�װ�á����ڸ�װ��Ϊ�ܱ�������������ú�ͣ���Ӧ������ӱ��ڹ۲죬���ɵ����ʱ��ڼ��飬����ˮ�ķ�Ӧ�����ƵĶ������ʣ�����ɫ��״̬���۵�ͺ��ܶ�С���ص㡣��λ��ˮ��ú�ͽ����ϣ���ˮ������Ӧ��2Na��2H2O===2NaOH��H2�������ɵ�H2������Ӧ��H2��CuO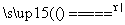 Cu��H2O������ˮ��ȫ��Ӧ��NaOH��Һ��
Cu��H2O������ˮ��ȫ��Ӧ��NaOH��Һ��
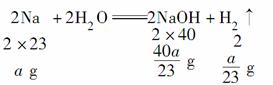
��w(NaOH)��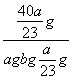 ��100%
��100%
��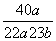 ��100%��
��100%��
���𰸡���(1)�۳�����ɫС����ˮ��ú�͵Ľ�������������������С
(2)���ϵ���������ɫ��Ϊ��ɫ��NaOH
(3)�ɺ�ɫ���ɫ��H2���ڵ��ܿڴ���ȼ���壬�е���ɫ�������
(4)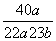 ��100%
��100%


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪8 g A����32 g Bǡ����ȫ��Ӧ������22 g C��һ����D���ֽ�16 g A��70 g B�Ļ�����ַ�Ӧ������2 mol D��һ������C����D��Ħ������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ƶõ�Na2S2O3�־��������������������ʡ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3��5H2O�ĺ�����һ�����������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ������ȡ1.28 g�Ĵ���Ʒ����ˮ����0.40 mol/L������ KMnO4��Һ�ζ�������Һ��S2O32��ȫ��������ʱ������KMnO4��Һ���20.00 mL��
�� �ζ��յ�ʱ��������________________________________________________________
�� д���õζ���Ӧ�����ӷ���ʽ__________________________________________________
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3��5H2O�����������IJⶨ���________(�ƫ�ߡ���ƫ�͡����䡱)
�ܾ����㣬��Ʒ��Na2S2O3��5H2O����������Ϊ ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡһС������Ʒ���ȼ�ճ��м��ȣ�����ʵ������������ȷ����(����)
�ٽ��������ۻ������ڿ�����ȼ�գ�������ɫ����
��ȼ�պ�ð�ɫ���塡��ȼ�պ����ɵ���ɫ��������
A���٢� B���٢ڢ�
C���ۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������¶���ڿ����У�����治�������ɵ�������(����)
A��Na2O B��NaOH
C��Na2CO3 D��NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȥCl2�е�����HCl���壬��ѡ��(����)
A��NaOH��Һ B��AgNO3��Һ
C������ʳ��ˮ D��ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��B��C�������壬A���ܶ���С�����壬B��ͨ������³ʻ���ɫ��������A������B�а�����ȼ������C��������Bͨ�뵽����ʯ�����п��Եõ���ɫ������D����ݴ˻ش��������⣺
(1)д�����и���Ӧ�Ļ�ѧ����ʽ��
�ٴ�����A��B�а�����ȼ������C��__________________________��
�ڽ�����Bͨ�뵽ˮ�У�_______________________________________��
�۽�����Bͨ�뵽NaOH��Һ�У�______________________________��
�ܽ�����Bͨ�뵽����ʯ�����У�________________________________��
(2)����������ֱ�ͨ��������������Һ�У����ְ�ɫ�����������ǣ�________(����ĸ��ʾ)��
(3)��ɫ������D�����Ư�����ֳ�Ϊ________���������ڿ��������ױ��ʵ�ԭ��Ϊ(�û�ѧ����ʽ��ʾ)________________ ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ԫ���У���һ�������ɴ�С˳����ȷ����
�٣�ԭ�Ӻ���δ�ɶԵ������ĵڶ�����Ԫ��
�ڣ������Ų�Ϊ1s2��Ԫ��
�ۣ����ڱ��е縺����ǿ��Ԫ��
�ܣ�ԭ�����������Ų�Ϊ3s23p4��Ԫ��
A.�ڢۢ٢� B.�ۢ٢ܢ� C.�٢ۢܢ� D.���Ƚ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��4NH3(g)+5O2(g)=4NO(g)+6H2O(g)������Ӧ���ʷֱ���v(NH3)��v(O2)��v(NO)��v(H2O) [��λ��mol/(L��s)]��ʾ������ȷ�Ĺ�ϵ�ǣ�( )
A.4/5v(NH3)=v(O2) B.5/6 v(O2)=v(H2O) C.2/3 v(NH3)=v(H2O) D.4/5 v(O2)=v(NO)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com