
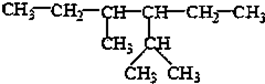
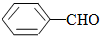
 ��ϵͳ����Ϊ2��4-����-3-�һ����飮
��ϵͳ����Ϊ2��4-����-3-�һ����飮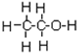 ��ϩ�ĵ���ʽ
��ϩ�ĵ���ʽ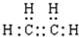
 ��
��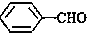
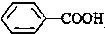 ��CH3COOH����ʾ����ȷӺʹ������ԣ�
��CH3COOH����ʾ����ȷӺʹ������ԣ� ���� ��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ���
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ���
�ڼ�Ϊ����ȥ��һ��Hԭ���γɵ�ԭ���ţ��Ҵ��к����ǻ�����ϩ�д���̼̼˫����
�ۼ�ȩ��ˮ��Һ��Ϊ�������֣������������ʽ��дͬ���칹�壬�м��ֻ�ѧ������ͬ��H���м��ַ壻
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ�
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������
��� �⣺��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
�ڼ�Ϊ����ȥ��һ��Hԭ�Ӻ�ʣ���ԭ���ţ��ṹ��ʽΪ��-CH3���Ҵ��к����ǻ����ṹʽΪ�� ����ϩ�д���̼̼˫��������ʽΪ��
����ϩ�д���̼̼˫��������ʽΪ��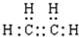 ���ʴ�Ϊ��-CH3��
���ʴ�Ϊ��-CH3�� ��
�� ��
��
�ۼ�ȩ��ˮ��Һ�׳Ƹ������֣�������������ụΪͬ���칹�壬�ṹ��ʽΪ��HCOOCH3���Զ�������������3�ֻ�ѧ������ͬ��H���ʺ˴Ź���������3��壬�ʴ�Ϊ����ȩ��HCOOCH3��3��
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ��������к���3��C��3���ǻ��� �к���3��C��2���ǻ����ʱ������ķе����
�к���3��C��2���ǻ����ʱ������ķе���� ���ʴ�Ϊ������
���ʴ�Ϊ������
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�����ˮ���ԣ�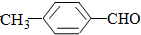 ��
�� ���ʴ�Ϊ����������
���ʴ�Ϊ����������
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������ʹ����������ӱ���������Ӹ��ȶ����������Ա�����ǿ���ʴ�Ϊ������
���� ������Ҫ��������л��������������ṹ��ʽ����д���л����۷е��Լ�����ǿ���ıȽϵȣ��ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
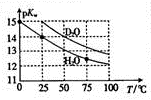 Hz0��D20��pKw ��pKw=-lgKw�����¶ȵĹ�ϵ��ͼ��ʾ�������й�˵����ȷ���ǣ�������
Hz0��D20��pKw ��pKw=-lgKw�����¶ȵĹ�ϵ��ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | D20�ĵ������Ϊ���ȹ��� | B�� | 25��ʱ����D20��pH����7 | ||
| C�� | 25��ʱ��pH=7��Һ��һ���Ǵ�H20 | D�� | �����£���Dz0�м���DCI��pKw��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
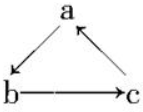 Na��Al��Fe��Cu����ѧ��ѧ����Ҫ�Ľ���Ԫ�أ����ǵĵ��ʼ��仯����֮���кܶ�ת����ϵ����ͨ����˵�ġ������ǡ����������ǡ��ȣ�����������ʲ��ܰ���ͼ����������ʾһ����ɣ���ϵ�ת�����ǣ�������
Na��Al��Fe��Cu����ѧ��ѧ����Ҫ�Ľ���Ԫ�أ����ǵĵ��ʼ��仯����֮���кܶ�ת����ϵ����ͨ����˵�ġ������ǡ����������ǡ��ȣ�����������ʲ��ܰ���ͼ����������ʾһ����ɣ���ϵ�ת�����ǣ�������| ѡ�� | A | B | C | D |
| a | NaOH | Al | Fe | Cu |
| b | Na | Al2O3 | FeCl3 | CuSO4 |
| c | NaCl | Al��OH��3 | FeCl2 | CuCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ͳ����ʽ�ζ��ܡ�ˮ | B�� | ����ƿ����ʽ�ζ��ܡ��� | ||
| C�� | ��Ͳ����ʽ�ζ��ܡ�ˮ | D�� | ����ƿ����ʽ�ζ��ܡ����Ȼ�̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
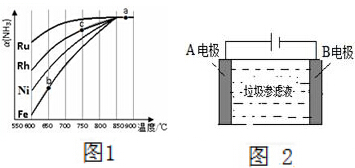 ����������������Ϳ�����Ӧ��ʮ�ֹ㷺��
����������������Ϳ�����Ӧ��ʮ�ֹ㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| H2CO3 | K${\;}_{{a}_{1}}$=4.3��10-7 | HClO | Ka=2.95��10-8 |
| K${\;}_{{a}_{2}}$=5.61��10-11 | AgCl | Ksp=1.77��10-10 | |
| CH3COOH | Ka=1.76��10-5 | Ag2CrO4 | Ksp=1.12��10-12 |
| I | �� | �� | �� | |
| FeCl3��Һ���/mL | 100 | 100 | 100 | 100 |
| �����ĩ����/g | 3 | 6.6 | 9 | 12 |
| ʣ���ĩ����/g | 0 | 0.64 | 3.2 | 6.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ba��OH��2��Һ�еμ�ϡ���Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O | |
| B�� | ���Խ�����KMnO4����H2O2��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O | |
| C�� | HCO3-�ĵ��뷽��ʽ��HCO3-+H2O�TH2CO3+OH- | |
| D�� | Cl2��H2O��Ӧ��Cl2+H2O�TH++Cl-+HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���� ���� | B�� | CH3CH��C2H5��CH2CH2CH3 2-�һ����� | ||
| C�� | 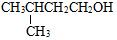 3-��-1-���� 3-��-1-���� | D�� | 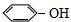 ʯ̼�� ʯ̼�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com