
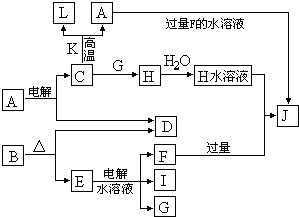
C��D��G��I��Ϊ������Ԫ���γɵĵ��ʣ�D��G��IΪ�����ǽ�����̬���ʡ�DԪ�ص�ԭ�ӵ������������Ǵ�����������3����C��Gͬ���ڣ���ԭ���������������4�����ǵļ����ӵ��Ӳ�ṹ��ͬ�����������ת����ϵ��
����գ�
(1) D��I���γ� ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ������д�������ʽ�� ��
ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ������д�������ʽ�� ��
��2��L��ĿǰӦ����㷺�Ľ�������̼����������L��������д�����Eˮ��Һ�Ļ�ѧʽ�� ��
��3��������K �к�����ɵ���L��Ԫ�أ��Ҹ�Ԫ�ص���������Ϊ70%����Ӧ�ٵĻ�ѧ����ʽ�� �������÷�Ӧ�IJ�����
��4��д��A+F �� J�����ӷ���ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����գ�?
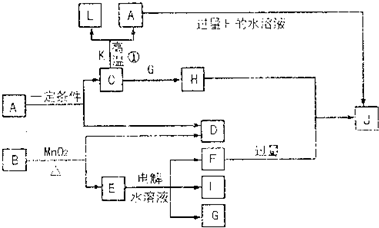
(1)D��Ԫ����I��Ԫ�����γ�ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ���������ʽΪ������������������?
(2)д����ʯī���缫�����Eˮ��Һ�����ӷ���ʽ����������������G�����������ݳ�(�����������)��?
(3)д��C+K��L+A�Ļ�ѧ����ʽ����������������ָ�������˷�Ӧ�ķ�������������������������������
(4)д��A+F��J�����ӷ���ʽ��������������������?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C��D��G��I��Ϊ������Ԫ���γɵĵ��ʣ�D��G��IΪ�����ǽ�����̬���ʡ�DԪ�ص�ԭ�ӵ������������Ǵ�����������3����C��Gͬ���ڣ���ԭ���������������4�����ǵļ����ӵ��Ӳ�ṹ��ͬ�����������ת����ϵ��
����գ�
(1) D��I���γ�ԭ�Ӹ�����Ϊ1��1�Ĺ��ۻ������д�������ʽ�� ��
��2��L��ĿǰӦ����㷺�Ľ�������̼����������L��������д�����Eˮ��Һ�Ļ�ѧʽ�� ��
��3��������K �к�����ɵ���L��Ԫ�أ��Ҹ�Ԫ�ص���������Ϊ70%����Ӧ�ٵĻ�ѧ����ʽ�� �������÷�Ӧ�IJ�����
��4��д��A+F ��J�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com