
����Ŀ����1����֪�������ԭ��������56 ����1����ԭ�ӵ�������_______g������NA��ʾ��
��2���ڱ�״���£�1.7g������ռ�����Ϊ_______L�������״����_____L���⺬����ͬ��Ŀ����ԭ�ӡ�
��3����֪CO��CO2�Ļ������������16.0g����״�������Ϊ8.96L�������֪��ȡ��������к�CO____g������CO2�ڱ긤״���µ����Ϊ__________L��
��4��ͬ��ͬѹ��ͬ�����H2��A����������ֱ���0.2g��l.6g��������A��ĦС����Ϊ________������A�ķ��Ӹ���Ϊ________������NA��ʾ��
��5����״���µ�aLHCl(g)����1000gˮ�У��õ��������ܶ�Ϊbg��cm-3�������������ʵ���Ũ����_____mol��L-1
���𰸡�![]() 2.24 3.36 2.8 6.72 16g/mol 0.1NA
2.24 3.36 2.8 6.72 16g/mol 0.1NA ![]()
��������
���⿼�鿼�������ʵ�����Ħ�����������ӵĸ���������Ħ����������ʵ���Ũ�ȹ�ʽ֮��Ļ��㣬�Լ�����٤�����ɵ�Ӧ�á�
��1���������ԭ��������56��������Ħ������Ϊ56g.mol-1��1molFe������Ϊ56g, 1molFeԭ�ӵĸ���ΪNA��������1������ԭ�ӵ�������![]() g��
g��
��2���ڱ�״���£�1.7g���������ʵ���Ϊ��![]() =0.1mol��V(NH3)= 0.1mol
=0.1mol��V(NH3)= 0.1mol![]() 22.4L.mol-1=2.24L����������ԭ�ӵ����ʵ���Ϊ��0.1
22.4L.mol-1=2.24L����������ԭ�ӵ����ʵ���Ϊ��0.1![]() 3=0.3mol����Ϊ�����к�����ԭ�ӵĸ��������⺬�е���ԭ�Ӹ�����ͬ��������n(H2S)
3=0.3mol����Ϊ�����к�����ԭ�ӵĸ��������⺬�е���ԭ�Ӹ�����ͬ��������n(H2S)![]() 2=0.3mol��n(H2S) =0.15mol��V(H2S)=0.15 mol
2=0.3mol��n(H2S) =0.15mol��V(H2S)=0.15 mol![]() 22.4L.mol-1=3.36L��
22.4L.mol-1=3.36L��
��3���ɷ������n(CO)+ n(CO2)= ![]() =0.4mol����28n(CO)+44n(CO2)=16.0g���ɵã�n(CO)=0.1mol��n(CO2)=0.3mol������m(CO)=0.1mol
=0.4mol����28n(CO)+44n(CO2)=16.0g���ɵã�n(CO)=0.1mol��n(CO2)=0.3mol������m(CO)=0.1mol![]() 28g.mol-1=2.8g��V(CO2)= 0.3mol
28g.mol-1=2.8g��V(CO2)= 0.3mol![]() 22.4L.mol-1=6.72L��
22.4L.mol-1=6.72L��
��4��ͬ��ͬѹ��ͬ�������H2��A��������ʵ�����ͬ��n(H2)= ![]() =0.1 mol��M(A)=
=0.1 mol��M(A)= ![]() =16g/mol��N(A)= 0.1NA
=16g/mol��N(A)= 0.1NA
��5����״���µ�aLHCl(g)�����ʵ���Ϊ![]() =
=![]() ����Һ������Ϊ
����Һ������Ϊ![]()
![]() 36.5 g/mol+1000g=(1000+
36.5 g/mol+1000g=(1000+![]() )g����Һ�����
)g����Һ�����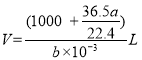 �������������ʵ���Ũ���ǣ�
�������������ʵ���Ũ���ǣ� =
=![]() mol��L-1��
mol��L-1��


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
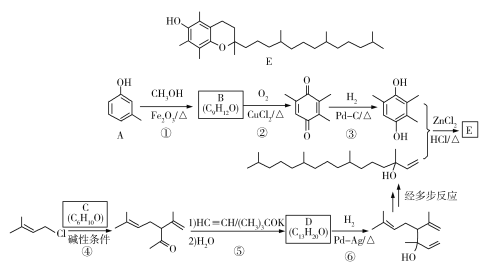
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]������J���Ʊ���Ѫ֬ҩ�������ص��м��壬�Ʊ�J��һ�ֺϳ�·��������£�

�ش��������⣺
(1)A�Ļ�ѧ������________________��
(2)G����H�ķ�Ӧ������________________��J�ķ���ʽΪ________________��
(3)C�Ľṹ��ʽΪ________________________��
(4)������I���ɷ��㻯����C6H6O�ͻ�״������C6H10O�ڴ������������Ʊ��õ����÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
(5)R��E��Ϊͬ���칹������E������ͬ�Ĺ������������Ŀ����R�Ľṹ��________�֡����ж�Ӧ�ĺ˴Ź�������ͼ��������壬�ҷ������Ϊ4��3��2��1�Ľṹ��ʽΪ________��
(6)������������(![]() )��һ��ũҩ������ɱ��Ӻ��Ҵ�Ϊ��ʼԭ���Ʊ��������������ĺϳ�·�ߣ�________________(���Լ���ѡ)��
)��һ��ũҩ������ɱ��Ӻ��Ҵ�Ϊ��ʼԭ���Ʊ��������������ĺϳ�·�ߣ�________________(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܱ������У���H2��COֱ���Ʊ������ѣ�CH3OCH3��������̰������·�Ӧ��
i.CO(g)+2H2(g)![]() CH3OH(g) H1=90.1kJ��mol1
CH3OH(g) H1=90.1kJ��mol1
ii.2CH3OH(g)![]() CH3OCH3(g)+H2O(g) H2=24.5kJ��mol1
CH3OCH3(g)+H2O(g) H2=24.5kJ��mol1
������������ͬʱ����H2��COֱ���Ʊ������ѵķ�Ӧ�У�COƽ��ת����������X ���仯��������ͼ��ʾ������˵����ȷ����
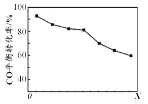
A . ��H2��COֱ���Ʊ������ѵķ�ӦΪ���ȷ�Ӧ
B. ����XΪѹǿ
C. X�������ѵIJ���һ������
D. X���÷�Ӧ��ƽ�ⳣ��һ����С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Goodenough������������ӵ�ؼ�����ﮡ�������﮵����������о������Խ�������2019��ŵ������ѧ�����ش��������⣺
(1)��̬Fe2+��Fe3+������δ�ɶԵĵ�����֮��Ϊ_________��
(2)Li�������ڱ�������Ԫ�صĵ�һ������(I1)�����ʾ��I1(Li)> I1(Na)��ԭ����_________��I1(Be)> I1(B)> I1(Li)��ԭ����________��
(3)��������ӵĿռ乹��Ϊ_______������P�ļ۲���Ӷ���Ϊ_______���ӻ��������Ϊ_______��
(4)LiFePO4�ľ����ṹʾ��ͼ��(a)��ʾ������OΧ��Fe��P�ֱ��γ�����������������壬����ͨ�������㡢�����γɿռ����ṹ��ÿ�������к���LiFePO4�ĵ�Ԫ����____����
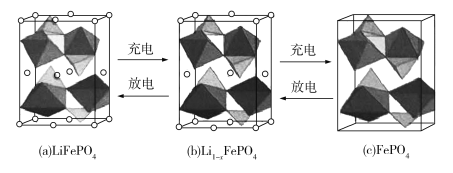
��س��ʱ��LiFeO4�ѳ�����Li+���γ�Li1xFePO4���ṹʾ��ͼ��(b)��ʾ����x=_______��n(Fe2+ )��n(Fe3+)=_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����±�ɫ���������ܵ���̫�������ʣ��Ӷ�ʵ�ֽ��ܡ���ͼ��ij���±�ɫ������ʾ��ͼ����ͨ��ʱ��Ag+ע�뵽��ɫWO3��Ĥ�У�����AgxWO3������������ɫ�����ڸñ仯���̣����������������
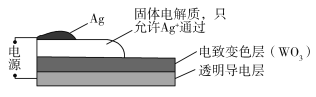
A.AgΪ����B.Ag+�����缫���ɫ��Ǩ��
C.WԪ�صĻ��ϼ�����D.�ܷ�ӦΪ��WO3+xAg=AgxWO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��NH3�ǹ�ҵ�����еij������壬�о����ڹ�ҵ�ϵķ�Ӧ���̶��������Ч����Ϊ��Ҫ��
I.��ҵ����CO��H2��ԭ�Ͽ��Ժϳɼ״�����ΪҺ��ȼ�ϡ���֪��
�� 2H2(g)��CO(g) ��![]() O2(g) = 2H2O(g)��CO2(g) ��H1= ��594.1kJ/mol
O2(g) = 2H2O(g)��CO2(g) ��H1= ��594.1kJ/mol
�� 2CH3OH(l)��3O2(g) = 4H2O(g)��2CO2(g) ��H2 = ��1452kJ/mol
��1����д����CO(g)��H2(g)�ϳ�1molҺ̬�״����Ȼ�ѧ��Ӧ����ʽ��______________��
��2��һ���¶��������ݻ�Ϊ2L�ĺ��������м���3mol H2��2mol CO������Ӧ2H2(g)��CO(g)![]() CH3OH(g)�ﵽƽ��ʱ����������ڵ�ѹǿ�Ƿ�Ӧǰѹǿ��
CH3OH(g)�ﵽƽ��ʱ����������ڵ�ѹǿ�Ƿ�Ӧǰѹǿ��![]() ������ø��¶��·�Ӧ��ƽ�ⳣ��K=____________�����ֺ��º��ݣ��������ﵽƽ�����������ͨ��CO(g)��CH3OH(g)��ʹ��CO(g)��CH3OH(g)Ũ�Ⱦ�Ϊԭƽ���2������ƽ���ƶ�����Ϊ________�ƶ�����������������������������������
������ø��¶��·�Ӧ��ƽ�ⳣ��K=____________�����ֺ��º��ݣ��������ﵽƽ�����������ͨ��CO(g)��CH3OH(g)��ʹ��CO(g)��CH3OH(g)Ũ�Ⱦ�Ϊԭƽ���2������ƽ���ƶ�����Ϊ________�ƶ�����������������������������������
II.���ڰ��ĺϳɷ�Ӧ N2(g)��3H2(g)![]() 2NH3(g)����H��0�����ܱ������ڳ���0.1mol/L N2��0.3 mol/L H2����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ���Իش����⣺
2NH3(g)����H��0�����ܱ������ڳ���0.1mol/L N2��0.3 mol/L H2����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ���Իش����⣺
��3�����������£��ӿ�ʼ��Ӧ��������ƽ��״̬��v(N2)��_________����Ӧ�ﵽƽ���5��
��ĩֻ�ı䷴Ӧ�¶ȣ����������������䣬��ı�������NH3�����ʵ���Ũ�Ȳ�����Ϊ_____��
A. 0.20 mol/L B. 0.12 mol/L C. 0.10 mol/L D. 0.08 mol/L
��4���ڵ�5����ʱ�������������Сһ�룬��Ӧ�ڵ�8����ʱ�ﵽ�µ�ƽ�⣬��ʱNH3��Ũ��ԼΪ0.30 mol/L��������ͼ�л�����5����֮���NH3Ũ�ȵı仯����______��
��5�������������䣬��ֻ��������Ϊ��ѹ����������0.2 molN2��0.6 molH2���ﵽƽ��ʱ��NH3���������Ϊm%�����������м�������0.2 molN2��0.6 molH2������ͬ�����¶��´ﵽƽ��ʱ��NH3���������Ϊn%����m��n�Ĺ�ϵ��ȷ����______��
A��m>n B��m<n C��m=n D�����Ƚ�%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ά����E��һ����������֬����ά���أ����ѹ㷺Ӧ����ҽҩ��Ӫ��Ʒ����ױƷ�ȡ���Ȼ��ά����E�ɶ�����������ɣ�������-������(������E)������ߣ���������Ҳ��ߡ������ǻ�����E��һ�ֺϳ�·�ߣ����в��ַ�Ӧ��ȥ��
��֪������Ϣ��a) 
b) 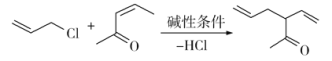
c) 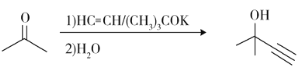
�ش��������⣺
(1)A�Ļ�ѧ����Ϊ_____________��
(2)B�Ľṹ��ʽΪ______________��
(3)��Ӧ��C��������������ṹ��ʽΪ______________��
(4)��Ӧ�ݵķ�Ӧ����Ϊ______________��
(5)��Ӧ�Ļ�ѧ����ʽΪ______________��
(6)������C��ͬ���칹������ͬʱ��������������������_________��(�����������칹�壬����)��
(��)������������(��)����ͪ�ʻ�(������C=C=O)��(��)�����л�״�ṹ��
(a)4 (b)6 (c)8 (d)10
���У���������̼(ע�������ĸ���ͬ��ԭ�ӻ���ŵ�̼)�Ļ�����Ľṹ��ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ�������Τ(Remdesivir)���¹ڲ����������������ã������� M�Ǻϳ������Τ���м��壬���й���M��˵���������
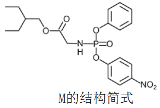
A.�˴Ź���������11�����շ�
B.�����к���3�ֺ���������
C.������Nԭ��һ����sp2�ӻ���һ����sp3�ӻ�
D.1mol������������NaOH��Һ��Ӧʱ����3molNaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
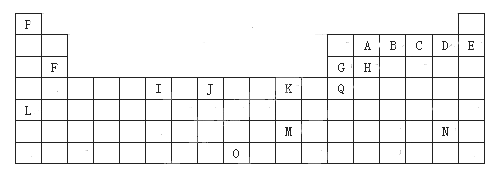
�Իش��������⣺
��1��I������ϼ�Ϊ___��I��Ԫ������Ϊ___��
��2��д����̬ʱKԪ��ԭ�ӵĵ����Ų�ʽ___��JԪ��ԭ�ӵ���Χ�����Ų�ʽ___��
��3�����жԱ���ȷ����___��
a.ԭ�Ӱ뾶H��G��B��A
b.��һ������E��D��C��B
c.�縺��A��H��G��Q
d.����������ˮ��������B��A��H��G
��4�����й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й���������ȷ����___��
a.Lλ��Ԫ�����ڱ��������ڢ�A�壬����s��Ԫ��
b.Oλ��Ԫ�����ڱ��������ڢ��壬����ds��Ԫ��
c.M����Χ�����Ų�ʽΪ6s1������ds��Ԫ��
d.H���������Χ�����Ų�ʽΪns2np2������p��Ԫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com