
25 ��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10��5 | K1��4.3��10��7 K2��5.6��10��11 | 3.0��10��8 |
��ش��������⣺
(1)CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ
________________________________________________________________________��
(2)ͬŨ�ȵ�CH3COO����HCO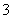 ��CO
��CO ��ClO�����H����������ǿ������˳��Ϊ________________________________________________________________________��
��ClO�����H����������ǿ������˳��Ϊ________________________________________________________________________��
(3)���Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000 mL��ϡ������pH�仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��______(����ڡ��������ڡ���С�ڡ�)����ĵ���ƽ�ⳣ����������__________________________________________________��
�𰸡�(1)CH3COOH>H2CO3>HClO
(2)CO >ClO��>HCO
>ClO��>HCO >CH3COO��
>CH3COO��
(3)���ڡ�ϡ����ͬ������HX��pH�仯��CH3COOH�Ĵ�����ǿ������ƽ�ⳣ����
����������ƽ�ⳣ��Խ������Խǿ������ƽ�ⳣ��ԽС�����Ӧ������ӽ��H������Խǿ��
(3)����ͼ�����֪������ʼʱ������Һ��c(H��)��ͬ����c(������)>c(��ǿ��)��ϡ�����н�����ĵ���̶�����Ķ࣬��������ϡ�����н������c(H��)һֱ���ڽ�ǿ���c(H��)��ϡ����ͬ������HX��pH�仯��CH3COOH�Ĵ�HX����ǿ������ƽ�ⳣ����


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
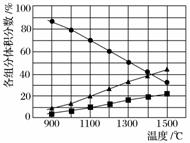
H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3��CuCl2�Ļ����Һ�м�����м����Ӧ�������˳��������ʣ���Һ�е������ӿ�����(����)
��ֻ��Fe2�������� ��Fe2����Fe3��
��Fe2����Cu2�������� ��Cu2����Fe3��
A���٢ۡ��� B���ڢܡ���
C���٢ܡ��� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ��ʾ��װ�ý���ʵ�顣
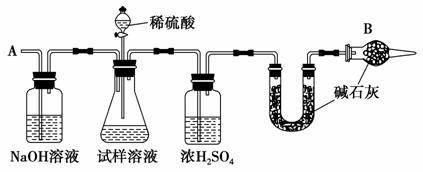
��Ҫʵ�鲽�����£�
�ٰ���ͼʾ��װ�����������װ�õ�������
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�۳���ʢ�м�ʯ�ҵ�U�ιܵ�����Ϊb g
�ܴӷ�Һ©������6 mol·L��1��ϡ���ᣬֱ�����ٲ�������Ϊֹ
�ݴӵ���A����������һ�����Ŀ���
���ٴγ���ʢ�м�ʯ�ҵ�U�ιܵ�����Ϊc g
���ظ�����ݺ͢IJ�����ֱ��U�ιܵ������������䣬Ϊd g
��ش��������⣺
(1)����������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��________________________________________________________________________��
(2)װ���и����B��������_____________________________________��
(3)�������Һ©���е����ỻ��Ũ����ͬ�����ᣬ�����Ľ����________(�ƫ�ߡ�����ƫ�͡����䡱)��
(4)����ݵ�Ŀ����________________________________________��
(5)����ߵ�Ŀ����_______________________________________________��
(6)�����д������������Ϊ________________(�ú�a��b��d�Ĵ���ʽ��ʾ)��
(7)������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ��ʵ�鷽��_______________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ����0.2 mol·L��1�Ĵ����ˮϡ�ͣ���ͼ�е�����y��ʾ����(����)
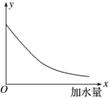
A����Һ��OH�������ʵ���Ũ��
B����Һ�ĵ�������
C����Һ�е�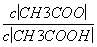
D��CH3COOH�ĵ���̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮϡ�ͣ�����ͼ�л���pHֵ�ı仯ͼ��
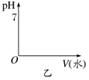
��ˮϡ����ͬ�ı�����________��pH��
��ˮϡ�͵���ͬ��pHֵ��________�����ˮ�ࡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij�¶���CH3COOH��NH3·H2O�ĵ��볣����ȣ�����10 mLŨ��Ϊ0.1 mol·L��1��CH3COOH��Һ�еμ���ͬŨ�ȵİ�ˮ���ڵμӹ�����(����)
A��ˮ�ĵ���̶�ʼ������
B.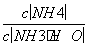 �������ټ�С
�������ټ�С
C��c(CH3COOH)��c(CH3COO��)֮��ʼ�ձ��ֲ���
D�������백ˮ�����Ϊ10 mLʱ��c(NH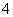 )��c(CH3COO��)
)��c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ1��ͼ2�ֱ���1s���ӵĸ��ʷֲ�ͼ��ԭ�ӹ��ͼ�������й���ʶ��ȷ����
(����)��

A��ͼ1�е�ÿ��С�ڵ��ʾ1������
B��ͼ2��ʾ1s����ֻ���������ڳ���
C��ͼ2����1s�����Բ�Σ��������Գ���
D��ͼ1�е�С�ڵ��ʾijһʱ�̣������ں���������λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����ʽΪC4H10O����������Ʒ�Ӧ�ų�H2���л���������________�֡�
(2)�뻯����C7H10O2��Ϊͬ���칹������ʲ�����Ϊ________��
A���� B��ȩ
C������ D����
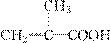 |
(3)�� ������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ
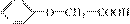 _______________��
_______________��
(4)��������( )�ж��������ͬ���칹�塣��������FeCl3��
Һ������ɫ��Ӧ���ұ�������2��һ����ȡ�����ͬ���칹����_________________(д������2�ֵĽṹ��ʽ)��
(5)����ʽΪC5H10��ϩ������(������˳���칹)________�֡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com