
���� ��1������������Һ���ѡ������ƿ�����1molNa2CO3•10H2O���ṩ1molNa2CO3���ó���Ҫ��Na2CO3•10H2O�����ʵ������ٸ���m=n•M����������������ʱ��Ϊ��ֹ��������ˮ���࣬����������ƿ�м�ˮ����1��2cmʱ�����ý�ͷ�ιܼ�ˮ���̶ȣ��Ӹ�ҡ�ȣ�
��2���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������0.2mol•L-1��Na2CO3��Һ480mL��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����ҪNa2CO3���ʵ���Ϊ��0.2mol•L-1��0.5L=0.1mol������1molNa2CO3•10H2O���ṩ1molNa2CO3����֪��Ҫ��Na2CO3•10H2O�����ʵ���n=0.1mol������m=n•M=0.1mol��286g/mol=28.6g������ʱ��Ϊ��ֹ��������ˮ���࣬����������ƿ�м�ˮ����1��2cmʱ�����ý�ͷ�ιܼ�ˮ���̶ȣ��Ӹ�ҡ�ȣ�
�ʴ�Ϊ��28.6g�� ��ͷ�ιܣ�
��2����̼���ƾ���ʧȥ�˲��ֽᾧˮ�����ʵ�����ƫ�����ʵ����ʵ���ƫ����������ҺŨ��ƫ�ߣ��ʢ�ѡ��
���á���������ij��������������壬���ʵ�����ƫ�ͣ����ʵ����ʵ���ƫ�ͣ���������ҺŨ��ƫ�ͣ��ʢڲ�ѡ��
��̼���ƾ��岻�������л����Ȼ��ƣ����ʵ�����ƫ�ͣ����ʵ����ʵ���ƫ�ͣ���������ҺŨ��ƫ�ͣ��ʢ۲�ѡ��
������ƿδ�������ʹ�ã����ʵ����ʵ�������Һ����������ı䣬����������ҺŨ�Ȳ��䣬�ʢܲ�ѡ��
��ѡ���٣�
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���������ǽ���ؼ����״����Ǽ������ʵ�������ע���������ķ�����


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
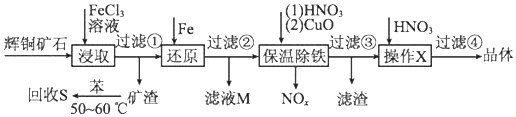
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4.7 | 2.7 | 7.6 |
| ��ȫ����pH | 6.7 | 3.7 | 9.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18gH2O����������Ϊ10NA | |
| B�� | ���³�ѹ�£�48gO3��O2����ԭ����Ŀ3NA | |
| C�� | 1molNa2O2������������ĿΪNA | |
| D�� | 11.2L����������ԭ����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��NH4��2Fe��SO4��2��Һ�м�������������ƣ�NH4++Fe2++30H-=NH3•H2O+Fe��OH��2�� | |
| B�� | ��������Һ�еμ�Ba��0H��2��Һ��SO42- ǡ����ȫ������2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� | |
| C�� | �����ʯ��ˮ�м������̼��������Һ��Ca2++2OH-+2HCO3-=CaCO3��+2H2O+CO32- | |
| D�� | ������FeC12��Һ�м���H2O2��2Fe2++2H2O2=2Fe3++O2��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
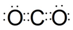 ���û������۷е����CS2��ԭ�������߶�Ϊ���Ӿ��壬�ҽṹ���ƣ�CS2ʽ������CO2��CS2���»�����CS2�۷е����CO2��
���û������۷е����CS2��ԭ�������߶�Ϊ���Ӿ��壬�ҽṹ���ƣ�CS2ʽ������CO2��CS2���»�����CS2�۷е����CO2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ѧ��Ӧ | |
| B�� | ����������������ԭ��Ӧ | |
| C�� | �����ױ����� | |
| D�� | ���ױ�����Ϊ��������������Ĥ���б����ڲ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | 25��ʱ����ͬŨ�ȵ�NaHCO3��Һ�ļ��Դ���NaClO��Һ | |
| B�� | ͼ����a��ĵ�������С��c�� | |
| C�� | ͼ�� I����CH3COOH��Һ��ϡ�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | �٢� | C�� | �٢ڢۢ� | D�� | �ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�������� | ��ʯ��ʯ��ϡ������ȡ CO2 | ���� | ��Һת�� |
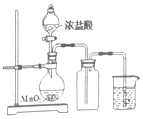 | 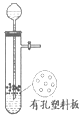 |  |  |
| A | B | C | D |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com