
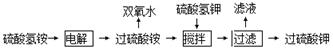

 (NH4)2S2O8��H2��2�֣�
(NH4)2S2O8��H2��2�֣� 2MnO4����10SO42����16H����2�֣�
2MnO4����10SO42����16H����2�֣� (NH4)2S2O8��H2����(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2����ʹ�ò���ԭ���ǣ�Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ���ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2���ٸտ�ʼ��S2O82��������Ӧ������H2O2 ����H2O2 �ֽ⣬��������FeCl3��Һ�����ǵ����˴������ӿ���H2O2 �ķֽ⣬����tʱ����Ѹ�ٽ��͡�������X����H2O2 ���ڵõ��Ϻ�ɫ��Һ�����и���������ɣ��ʷ�Ӧ����ʽΪ2Mn2����5S2O82����8H2O
(NH4)2S2O8��H2����(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2����ʹ�ò���ԭ���ǣ�Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ���ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2���ٸտ�ʼ��S2O82��������Ӧ������H2O2 ����H2O2 �ֽ⣬��������FeCl3��Һ�����ǵ����˴������ӿ���H2O2 �ķֽ⣬����tʱ����Ѹ�ٽ��͡�������X����H2O2 ���ڵõ��Ϻ�ɫ��Һ�����и���������ɣ��ʷ�Ӧ����ʽΪ2Mn2����5S2O82����8H2O  2MnO4����10SO42����16H��
2MnO4����10SO42����16H��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
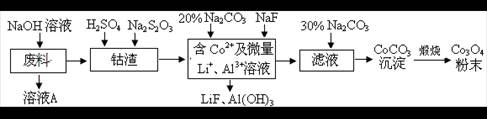
 LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(1)(3)(4)(5) | B��(2)(3)(5)(6) | C��(3)(4)(5)(6) | D��(1)(2)(3)(4) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������������������ά | B������ʦ���������ʴʯӢ��������Ʒ |
| C��ˮ�������Ͳ����ϵĴ��̶��ǹ�������Ʒ | D���ֹ��Ʊ������費�漰������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������һ������Ʒ��ʹ�� |
| B������̫��������ˮ���к�ˮ�ĵ��� |
| C���ܿ�������ͨ��ӵ���������Լݳ��� |
| D����ˮ�͵�ˮ���㴦��װ��Ĥ������ˮ�е����Ũ�ȵIJ�����з��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
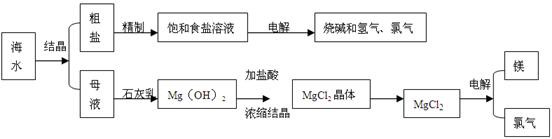
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na | B��Mg | C��Ag | D��Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
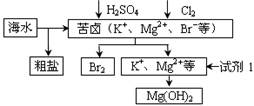
| A������BaCl2��Һ��ȥ�����е�SO42- |
| B���ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br- + Cl2 ="=" 2Cl- + Br2 |
| C���Լ�1����ѡ��ʯ���� |
| D����ҵ�ϣ��������Mg(OH)2ұ������þ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
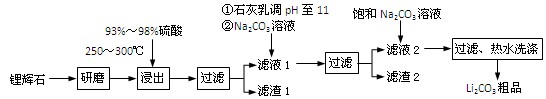
 H2SO4��Ũ��
H2SO4��Ũ�� Li2SO4
Li2SO4 Al2O3��4SiO2��H2O��
Al2O3��4SiO2��H2O��| T/�� | 20 | 40 | 60 | 80 |
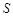 (Li2CO3)/g (Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
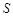 (Li2SO4)/g (Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com