
��1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
��1��1000:1 ��2��2 2��10�C2 ��3�� A ��4��10�C13 107:1 6.5
��5��5.0��10�C7 mol��L�C1 5.0��10�C11 mol��L�C1 5.0��10�C12 mol��L�C1
���������������1��c(Na+)��c(Cl�C)��1��10��4 mol��L�C1��c(OH�C)��1��10��7mol��L�C1��c(Na��)��c(OH��)��1��10��4��1��10��7��1000:1����2����NaOH��Һ��H2SO4��Һ�������ΪV�������ڷ�Ӧ��H2SO4����������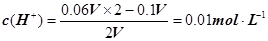 ����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4 mol��L�C1���������Ϻ�Ļ����Һ��
����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4 mol��L�C1���������Ϻ�Ļ����Һ��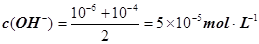 ��������Ũ��Ϊ
��������Ũ��Ϊ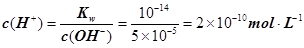 ����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4�� ����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)=
����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4�� ����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)= 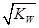 = 10�C6.5 mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)=5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C) =Kw/c(OH�C)=5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)=Kw/c(H+)=5.0��10�C12 mol��L�C1��
= 10�C6.5 mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)=5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C) =Kw/c(OH�C)=5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)=Kw/c(H+)=5.0��10�C12 mol��L�C1��
���㣺������Һ������Ũ�ȼ��㡢pH���㡢ˮ�����ӻ��ļ��㡣


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪H2A��ˮ�д�������ƽ�⣺H2A H����HA����HA��
H����HA����HA�� H����A2�����ش��������⣺
H����A2�����ش��������⣺
��1����֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺
CaA(s) Ca2��(aq)��A2��(aq)����H �� 0��
Ca2��(aq)��A2��(aq)����H �� 0��
���¶�����ʱ��Ksp________(���������С�����䡱��ͬ)��
�ڵμ�����Ũ���ᣬc (Ca2��)________��ԭ����____ ______________(�����ֺ����ӷ���ʽ˵��)��
��2������CaA����Һ�м���CuSO4��Һ������һ�ֺ�ɫ�������ʣ�д���ù����з�Ӧ�����ӷ���ʽ______________________����ijCuSO4��Һ��c (Cu2��)��0.02 mol/L�����Ҫ����Cu(OH)2������Ӧ������ҺpH��ʹ֮����________(��֪Ksp[Cu(OH)2]��2.0��10��20)��
��3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ��________�ԡ��ڷ�������Һ����̪�ʺ�ɫ��ԭ��ʱ����ͬѧ��Ϊ��������Һʱ���õĴ�����Ʒ�л���NaOH���£���ͬѧ��Ϊ����Һ�е������CO32-ˮ�����£��������һ����ʵ�鷽����������λͬѧ��˵����������(������Ҫ����������ͽ���)_________________ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ���ڵ���ƽ�⣬H2O H++OH? ����ش�
H++OH? ����ش�
���������¶ȣ�����̶Ƚ� ����Һ�� �ԡ�
�������м�������ϡ��ˮ��ˮ�ĵ���ƽ�� �ƶ�����Һ��PH ��
���������м�������NaHSO4��Һ��ˮ�����ӻ����� ����ˮ�������c(H+) c(OH_)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȡ���100��ʱ1 mol��L��1��NaOH��Һ�У���ˮ�������c(H��)��___________mol��L��1��25 ��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ����________(��ٽ����������ơ���Ӱ�족)��
(2)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݡ�
| ��ѧʽ | ����ƽ�ⳣ��(25��) |
| HCN | K��4.9��10��10 |
| CH3COOH | K��1.8��10��5 |
| H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ȱ�ʾ����ʵ����ǿ��������ȵĶ��壺
�������ѵ���ĵ���ʷ���������Һ��ԭ�е���ʵ��ܷ���������100%��
��֪25��ʱ��������(��)�ĵ����(��ҺŨ�Ⱦ�Ϊ0.1 mol��L��1)���±���
| ��� | ����(��) | ����Ȧ� |
| A | ������Һ(��һ����ȫ����)�� �ڶ��� HSO4- H����SO42- H����SO42- | 10% |
| B | ����������Һ�� HSO4- H����SO42 H����SO42 | 29% |
| C | ��� CH3COOH CH3COO����H�� CH3COO����H�� | 1.33% |
| D | ��� HCl��H����Cl�� | 100% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ӻ�ˮ����ȡþ������������þ����Ҫ��Դ����������ȡþ�Ĺ������漰�ļ������ʵ��ܶȻ�����������ѧ��֪ʶ�ش����м������⣺
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 |
| �ܶȻ� | 2.8��10�C9 | 6.8��10�C6 | 5.5��10�C6 | 1.8��10�C11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���仯�����й㷺��Ӧ�ã���SO2���ʵ��о��Ǹ��л�ѧ��ѧ��һ����Ҫ���ݡ�
I���Ա��о���һ����Ҫ���о�������������ĵ��ʼ����ֻ����ﰴ���±���ʾ�ֳ�3 �飬���2��������M�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S (����) | SO2��H2SO3��M��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |

| n(SO32��)��n(HSO3��) | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| ������ | ����һ����Һ�м���KMnO4��Һ��Һ | �Ϻ�ɫ��ȥ | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ǿ�ᣬ��ѧ�ν�������ˮ��Һ�п�����ȫ���롣����ʵ�ǣ�������ˮ�еĵ�һ����������ȫ�ģ��ڶ������벢����ȫ����������Ϊ:H2SO4=H++HSO4-��HSO4- H+ + S042-��
H+ + S042-��
��ش������й�����:
��1��Na2SO4��Һ��_(������ԡ��������ԡ��������ԡ�)����������_
(�����ӷ���ʽ��ʾ)��
��2��H2SO4��Һ��BaC12��Һ��Ӧ�����ӷ���ʽΪ_ ��
��3����0��l0mol��L-1��Na2SO4��Һ�У���������Ũ�ȹ�ϵ��ȷ����_ (��д���)��
| A��c(Na+)=c(SO42-)+c��HSO4һ)+c(H2SO4) |
| B��c(OH-)="c(" HSO4-)+c(H+) |
| C��c( Na+)+c(H+)=c(OH-)+c(HSO4-)+2c(SO42-) |
| D��c( Na+)=2c(SO42-)+2c(HSO4-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ѧ�������֮����ת�����˵�����ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ����(��ͼ1)����Ӧԭ��Ϊ��2Na��FeCl2  Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
���ʱ��__________(д��������)�缫�ӵ�Դ�ĸ�����
�õ�صĵ����Ϊ________ _��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ(��ͼ2)��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������ ������ (�������ԭ��)��Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫����Ӧʽ�ǣ�
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������
��3������ʱ��BaSO4��Ksp��1.08��10-10,�ֽ��������BaCl2��Һ��2.0��10-3mol/l��Na2SO4
��Һ��ϡ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com