
 ��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ��ijѧϰС�����������װ��������ʵ�顣��ش�������⡣
��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ��ijѧϰС�����������װ��������ʵ�顣��ش�������⡣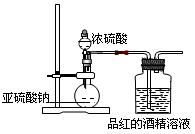
| ͬŨ����Һ | SO32- | HSO3- | H2SO 3 3 | ��ɫ�ٶ� |
| Na2SO3��Һ | �� | �� | �� | �� |
| NaHSO3��Һ | ������ | ������ | ������ | �� |
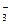 ��SO
��SO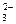 ��3�֣�
��3�֣�| ͬŨ����Һ | SO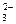 | HSO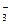 | H2SO3 | ��ɫ�ٶ� |
| Na2SO3��Һ | �� | �� | ���� | �� |
| Na HSO3��Һ | �������� | ������� | ������� | �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4��2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | �� |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g��cm-3�� | 1.900 | 1.650 |
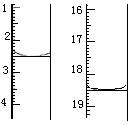
 ����157��ʱ��������������ʼ�ֽ⡣����Ʋ�����ˮ���������������ṩ����Ϣ���ش��������⣺
����157��ʱ��������������ʼ�ֽ⡣����Ʋ�����ˮ���������������ṩ����Ϣ���ش��������⣺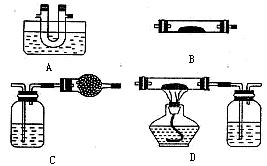
| A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ |
| B���ⶨ��ͬŨ�ȵIJ����������Һ��pH |
| C���ⶨ�����ƣ�Na2C2O4����Һ��pH |
| D����������Һ����Na2CO3��Һ�У���CO2�ų� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| ʵ�� | ʵ������ | ���� |
| A | ����ձ��������������ݣ��ұ��ձ���ͭ���������� | ��ԣ�Al��Fe��Cu |
| B | ��ƿ����ɫ��dz��ͬ | ˵����2NO2 N2O4��g�� ��H��0 |
| C | ��ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ | �ܶȻ���Ksp����AgCl��AgBr ��Ag2S |
| D | ��ƿ��������������ձ���Һ������ | �ǽ����ԣ�Cl��C��Si |
�鿴�𰸺ͽ���>>
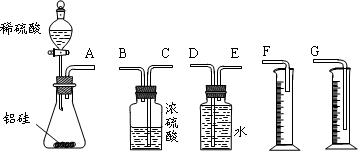
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
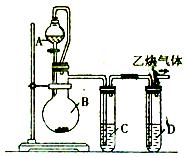
 ��ѧ����ʽ��__________________________��
��ѧ����ʽ��__________________________�� ____________��
____________�� Ϊ�˲�����������Ȳ������������С��ͬѧ�������ͼ��ʾ������װ�á���Ӧѡ��װ��_______
Ϊ�˲�����������Ȳ������������С��ͬѧ�������ͼ��ʾ������װ�á���Ӧѡ��װ��_______ __�������
__�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
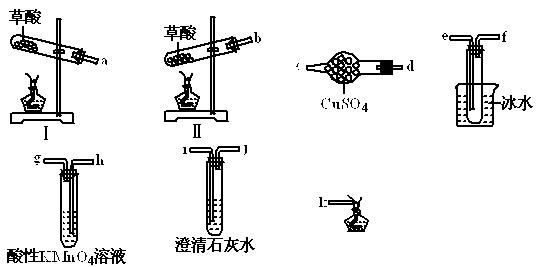
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ʵ���������µ�������ҩƷ�ɹ�ʹ�ã�
ʵ���������µ�������ҩƷ�ɹ�ʹ�ã�

 ��ش�
��ش�

| ��� | ʵ����� | Ԥ������ͽ��� |
| �� |   | ����ɫ���������˵����Ʒ�к���Na2CO3�� �������������˵����Ʒ��û��Na2CO3�� |
| �� |   | |
| �� |   | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

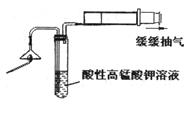
 �ص�������Ҫ��Ӧ�ķ���ʽ�� ��
�ص�������Ҫ��Ӧ�ķ���ʽ�� ��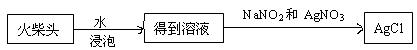
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com