
¢ń.ÓƵ¼Ļß½«A”¢BĮ½×°ÖƵÄĖÄøöµē¼«½ųŠŠĮ¬½Ó£¬Ź¹a¼«Īö³öĶ”£»Ų“šÓŠ¹ŲĪŹĢā”£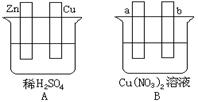
£Ø1£©µ¼Ļß½«ČŻĘ÷AŗĶBĮ¬½ÓŹ±£¬Zn½Ó £¬Cu½Ó £ØĢī”°a”±»ņ”°b”±£©
£Ø2£©ČŻĘ÷AÖŠCu¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ ”£
£Ø3£©B×°ÖĆ½Š £¬ČÜŅŗÖŠµÄNO3-Ļņ_____¼«ŅĘ¶Æ£ØĢī”°a”±»ņ”°b”±£©”£
£Ø4£©Čōb¼«¹Ū²ģµ½ÓŠĪŽÉ«ĪŽĪ¶ĘųÅŻ²śÉś, ¾¹żŅ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹·“Ó¦²¢½Į°č¾łŌČ£¬ČÜŅŗµÄpHÖµ½« £ØĢī”°Éżøß”±”¢”°½µµĶ”±»ņ”°²»±ä”±£©£¬¼ÓČėŅ»¶ØĮæµÄ ŗó£ØĢī»ÆѧŹ½£©£¬ČÜŅŗÄÜ»Öø“ÖĮÓė·“Ó¦Ē°ĶźČ«Ņ»ÖĀ”£Čō·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬲āµĆČÜŅŗÖŠCu2+ÅضČƻӊĆ÷ĻŌĻĀ½µ£¬æÉÄܵÄŌŅņŹĒ£ŗ ”£
¢ņ.³“²ĖµÄĢś¹ųƻӊĻ“øɾ»ČŻŅ×ÉśŠā”£ÓƱŲŅŖµÄĪÄ×ÖŗĶÓŠ¹Ų»Æѧ·½³ĢŹ½ĖµĆ÷ĢśŠāŹĒČēŗĪŠĪ³ÉµÄ
ӣ
¢ń.£Ø1£©a£¬b £Ø2£©2H+ + 2e- = H2”ü£Ø3£©µē½ā³Ų£¬b£Ø»ņŃō£©
£Ø4£©½µµĶ£¬CuO b¼«²ÄĮĻĪŖCu
¢ņ.Ģś¹ųŹĒÉśĢśÖĘŌģµÄ£¬ÉśĢśÖŠµÄFe”¢CŗĶ³“²ĖĪ“Ļ“¾»µÄŹ³ŃĪĖ®¹¹³ÉŌµē³Ų£Ø1·Ö£©£¬Ź¹Ģś·¢Éśµē»ÆѧøÆŹ“Éś³ÉFe(OH)2£¬ Fe(OH)2ŌŁ±»Ńõ»Æ³ÉFe(OH)3£¬Fe(OH)3²æ·ÖŹ§Ė®µĆµ½ĢśŠā”£øŗ¼«£ŗ2Fe - 4e- = 2Fe2+£»Õż¼«£ŗO2 + 2H2O +4e- = 4OH-£»
4Fe(OH) +O2 +2H2O = 4Fe(OH)3
½āĪöŹŌĢā·ÖĪö£ŗI.AæÉŠĪ³ÉŌµē³Ų£¬Zn×÷øŗ¼«£¬Cu×÷Õż¼«£¬Ź¹a¼«Īö³öĶ£¬a¼«ĪŖŅõ¼«£¬ĖłŅŌaÓėZnĮ¬½Ó£¬bÓėCuĮ¬½Ó”£AÖŠCu¼«·¢ÉśµÄ·“Ó¦ĪŖ2H++2e-=H2”ü”¢Zn¼«·¢ÉśµÄ·“Ó¦ĪŖZn-2e-=Zn2+”£B×°ÖĆ½Šµē½ā³Ų£¬ČÜŅŗÖŠNO3-ĻņŃō¼«ŅĘ¶Æ”£µē½āĻõĖįĶČÜŅŗÉś³ÉĶ”¢ĻõĖįŗĶŃõĘų£¬ČÜŅŗĖįŠŌŌöĒ棬pH¼õŠ”£¬Ńō¼«Īö³öŃõĘų”¢Ņõ¼«Īö³öĶ£¬¼ÓČėŃõ»ÆĶÄÜŹ¹ČÜŅŗÅØ¶Č»Öø“µ½·“Ó¦Ē°ÅØ¶Č£¬Čō·“Ó¦Ņ»¶ĪŹ±¼äŗóCu2+ÅضČƻӊĆ÷ĻŌĻĀ½µ£¬ĖµĆ÷Ńō¼«ČܽāCuÉś³ÉCu2+”£II.ĢśÉśŠāµÄ»śĄķŹĒĢś”¢Ģ攢Ź³ŃĪĖ®ŠĪ³ÉŌµē³Ų£¬FeĪŖøŗ¼«£¬Fe-2e-=Fe2+”£ĢæĪŖÕż¼«£¬µē¼«·“Ó¦ĪŖO2+2H2O+4e-=4OH-£¬×Ü·“Ó¦ĪŖ2Fe+O2+2H2O=2Fe(OH)2£¬Fe(OH)2±»Ńõ»ÆĪŖFe(OH)3”¢Fe(OH)3Ź§Ė®Éś³ÉFe2O3”¤xH2O”£
æ¼µć£ŗµē»Æѧ
µćĘĄ£ŗøÖĢśµē»ÆøÆŹ“·ÖĮ½ÖÖ£ŗĪüŃõøÆŹ“ŗĶĪöĒāøÆŹ“£¬µ±µē½āÖŹČÜŅŗĖįŠŌ½ĻĒæŹ±£¬·¢ÉśĪöĒāøÆŹ“£»µ±µē½āÖŹČÜŅŗ³ŹÖŠŠŌ»ņ¼īŠŌŹ±·¢ÉśĪüŃõøÆŹ“£¬



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
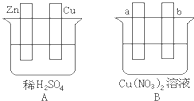 ¢ń£®ÓƵ¼Ļß½«A”¢BĮ½×°ÖƵÄĖÄøöµē¼«½ųŠŠĮ¬½Ó£¬Ź¹a¼«Īö³öĶ£®»Ų“šÓŠ¹ŲĪŹĢā£®
¢ń£®ÓƵ¼Ļß½«A”¢BĮ½×°ÖƵÄĖÄøöµē¼«½ųŠŠĮ¬½Ó£¬Ź¹a¼«Īö³öĶ£®»Ų“šÓŠ¹ŲĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ¹ć¶«Ź”ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌĄķæĘ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¢ń.ÓƵ¼Ļß½«A”¢BĮ½×°ÖƵÄĖÄøöµē¼«½ųŠŠĮ¬½Ó£¬Ź¹a¼«Īö³öĶ”£»Ų“šÓŠ¹ŲĪŹĢā”£

£Ø1£©µ¼Ļß½«ČŻĘ÷AŗĶBĮ¬½ÓŹ±£¬Zn½Ó £¬Cu½Ó £ØĢī”°a”±»ņ”°b”±£©
£Ø2£©ČŻĘ÷AÖŠCu¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ ”£
£Ø3£©B×°ÖĆ½Š £¬ČÜŅŗÖŠµÄNO3-Ļņ_____¼«ŅĘ¶Æ£ØĢī”°a”±»ņ”°b”±£©”£
£Ø4£©Čōb¼«¹Ū²ģµ½ÓŠĪŽÉ«ĪŽĪ¶ĘųÅŻ²śÉś, ¾¹żŅ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹·“Ó¦²¢½Į°č¾łŌČ£¬ČÜŅŗµÄpHÖµ½« £ØĢī”°Éżøß”±”¢”°½µµĶ”±»ņ”°²»±ä”±£©£¬¼ÓČėŅ»¶ØĮæµÄ ŗó£ØĢī»ÆѧŹ½£©£¬ČÜŅŗÄÜ»Öø“ÖĮÓė·“Ó¦Ē°ĶźČ«Ņ»ÖĀ”£Čō·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬲āµĆČÜŅŗÖŠCu2+ÅضČƻӊĆ÷ĻŌĻĀ½µ£¬æÉÄܵÄŌŅņŹĒ£ŗ ”£
¢ņ.³“²ĖµÄĢś¹ųƻӊĻ“øɾ»ČŻŅ×ÉśŠā”£ÓƱŲŅŖµÄĪÄ×ÖŗĶÓŠ¹Ų»Æѧ·½³ĢŹ½ĖµĆ÷ĢśŠāŹĒČēŗĪŠĪ³ÉµÄ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”¹ćÖŻŹŠÖ“ŠÅ֊ѧø߶ž£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£ØĄķ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com