
¾ŪŗĻĮņĖįĢśÓÖ³Ę¾ŪĢś£¬»ÆѧŹ½ĪŖ[Fe2(OH)n(SO4)3£0.5n]m£¬¹ć·ŗÓĆÓŚĪŪĖ®“¦Ąķ”£ŹµŃéŹŅĄūÓĆĮņĖį³§ÉÕŌü(Ö÷ŅŖ³É·ÖĪŖĢśµÄŃõ»ÆĪļ¼°ÉŁĮæFeS”¢SiO2µČ)Öʱø¾ŪĢśŗĶĀĢ·Æ(FeSO4·7H2O)£¬¹ż³ĢČēĻĀĖłŹ¾£ŗ

(1)ŃéÖ¤¹ĢĢåW±ŗÉÕŗó²śÉśµÄĘųĢåÖŠŗ¬ÓŠSO2µÄ·½·ØŹĒ
________________________________________________________________________ӣ
(2)ÖʱøĀĢ·ÆŹ±£¬ĻņČÜŅŗXÖŠ¼ÓČė¹żĮæ__________£¬³ä·Ö·“Ó¦ŗ󣬾__________²Ł×÷µĆµ½ČÜŅŗY£¬ŌŁ¾ÅØĖõ”¢½į¾§µČ²½ÖčµĆµ½ĀĢ·Æ”£
(3)ČÜŅŗZµÄpHÓ°Ļģ¾ŪĢśÖŠĢśµÄÖŹĮæ·ÖŹż£¬ÓĆpHŹŌÖ½²ā¶ØČÜŅŗpHµÄ²Ł×÷·½·ØĪŖ________________________________________________________________________”£
ČōČÜŅŗZµÄpHĘ«Š”£¬½«µ¼ÖĀ¾ŪĢśÖŠĢśµÄÖŹĮæ·ÖŹż________(Ģī”°Ę«“ó”±»ņ”°Ę«Š””±)”£
“š°ø””(1)½«²śÉśµÄĘųĢåĶØČėŹ¢ÓŠĘ·ŗģČÜŅŗµÄŠ”ŹŌ¹ÜÖŠ£¬ČōĘ·ŗģČÜŅŗĶŹÉ«£¬¼ÓČČÄÜ»Öø“ŌÉ«£¬Ö¤Ć÷ĘųĢåÖŠŗ¬ÓŠSO2
(2)ĢśŠ¼””¹żĀĖ
(3)Č”Ņ»Š”æépHŹŌÖ½£¬·ÅŌŚøÉŌļ”¢½ą¾»µÄ±ķĆęĆó(»ņ²£Į§Ę¬)ÉĻ£¬ÓĆøÉŌļ”¢½ą¾»µÄ²£Į§°ōÕŗČ”“ż²āČÜŅŗ£¬µĪµ½pHŹŌÖ½ÉĻ£¬Č»ŗóÓė±ź×¼±ČÉ«æØ±Č½Ļ””Ę«Š”
½āĪö””(1)ĄūÓĆSO2µÄĘư׊ŌĄ“¼ų±š¶žŃõ»ÆĮņ”£
(2)ČÜŅŗÖŠÓŠFe3£«”¢Fe2£«£¬ÓūÖĘĀĢ·Æ£¬Ó¦°ŃFe3£«×Ŗ»ÆĪŖFe2£«£¬ĖłŅŌŅŖ¼Ó¹żĮæµÄĢśŠ¼”£
(3)ÓĆpHŹŌÖ½²ā¶ØČÜŅŗµÄpH£¬ĪŖĮĖ²ā¶ØµÄ×¼Č·ŠŌ£¬Ó¦Ń”ÓĆøÉŌļ”¢½ą¾»µÄ×°ÖĆ”£ČōpHĘ«Š”£¬ĖįŠŌ¹żĒ棬ŌņFe3£«ÄŃŅŌŠĪ³É¾ŪĢś½ŗĢ壬ĖłŅŌ¾ŪĢśÖŠĢśµÄÖŹĮæ·ÖŹżĘ«Š””£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)±ūĻ©µÄ½į¹¹¼ņŹ½£ŗC3H6(””””)
(2013·½ĖÕ£¬2A)
(2)½«Ä³ĘųĢåĶØČėäåĖ®ÖŠ£¬äåĖ®ŃÕÉ«ĶŹČ„£¬øĆĘųĢåŅ»¶ØŹĒŅŅĻ©(””””)
(2013·½ĖÕ£¬13C)
(3)ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ų±š±½”¢»·¼ŗĻ©ŗĶ»·¼ŗĶé(””””)
(2013·ŗ£ÄĻ£¬7D)
(4)»·ĪģĶé( )ŌŚ¹āÕÕĻĀÓėĀČĘų·“Ó¦£¬Ö»Éś³ÉŅ»ÖÖŅ»ĀČ“śĪļ(””””)
)ŌŚ¹āÕÕĻĀÓėĀČĘų·“Ó¦£¬Ö»Éś³ÉŅ»ÖÖŅ»ĀČ“śĪļ(””””)
(2013·ŗ£ÄĻ£¬9Bøıą)
(5)ŅŅĻ©”¢¾ŪĀČŅŅĻ©ŗĶ±½·Ö×ÓÖŠ¾łŗ¬ÓŠĢ¼Ģ¼Ė«¼ü(””””)
(2013·ø£½ØĄķ×Ū£¬7C)
(6)ĪģĶé(C5H12)ÓŠĮ½ÖÖĶ¬·ÖŅģ¹¹Ģå(””””)
(2013·ø£½ØĄķ×Ū£¬7B)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻ£¬ĶعżČēĻĀ×Ŗ»ÆæÉÖʵĆKClO3¾§Ģå£ŗ
NaClČÜŅŗ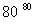 NaClO3ČÜŅŗ
NaClO3ČÜŅŗ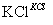 KClO3¾§Ģå
KClO3¾§Ģå
¢ŁĶź³É¢ńÖŠ·“Ó¦µÄ×Ü»Æѧ·½³ĢŹ½£ŗNaCl£«H2O===NaClO3£«__________”£
¢Ś¢ņÖŠ×Ŗ»ÆµÄ»ł±¾·“Ó¦ĄąŠĶŹĒ________£¬øĆ·“Ó¦¹ż³ĢÄÜĪö³öKClO3¾§Ģå¶ųĪŽĘäĖū¾§ĢåĪö³öµÄŌŅņŹĒ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×”¢ŅŅĮ½Ķ¬Ń§ŃŠ¾æNa2SO3ČÜŅŗÓėFeCl3ČÜŅŗ·“Ó¦µÄĒéæö”£
| ²½Öč | ²Ł×÷ | ĻÖĻó |
| ¢ń | Ļņ2 mL 1 mol·L£1FeCl3ČÜŅŗÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO3ČÜŅŗ | ČÜŅŗÓÉ×Ų»ĘÉ«±äĪŖŗģŗÖÉ«£¬²¢ÓŠÉŁĮæ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢåŅŻ³ö |
(1)³£ĪĀĻĀ£¬FeCl3ČÜŅŗµÄpH________7(Ģī”°<”±”¢”°>”±»ņ”°£½”±)”£
(2)·ÖĪöŗģŗÖÉ«²śÉśµÄŌŅņ”£
¢Ł¼×Ķ¬Ń§ČĻĪŖ²½Öč¢ńÖŠČÜŅŗ³ŹŗģŗÖÉ«ŹĒŅņĪŖÉś³ÉĮĖFe(OH)3£¬ÓĆ»ÆŃ§Ę½ŗāŅʶÆŌĄķ½āŹĶČÜŅŗ³ŹŗģŗÖÉ«µÄŌŅņ£ŗ________________________________________________________________________”£
¢ŚŅŅĶ¬Ń§ČĻĪŖæÉÄÜŹĒ·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦£¬Ķź³É²¢ÅäĘ½Ęä·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
 Fe3£«£«
Fe3£«£« SO
SO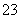 £«
£« ________——
________—— Fe2£«£«
Fe2£«£« ________£«
________£« ________
________
 ŅŅĶ¬Ń§²éŌÄ׏ĮĻµĆÖŖ£ŗ
ŅŅĶ¬Ń§²éŌÄ׏ĮĻµĆÖŖ£ŗ
ⅰ.Fe2£«Óė ·“Ӧɜ³ÉÄ«ĀĢÉ«µÄŠõד³ĮµķFeSO3£»
·“Ӧɜ³ÉÄ«ĀĢÉ«µÄŠõד³ĮµķFeSO3£»
ⅱ.Ä«ĀĢÉ«µÄFeSO3Óė»ĘÉ«µÄFeCl3ČÜŅŗ»ģŗĻŗó£¬ČÜŅŗ³ŹŗģŗÖÉ«”£
(3)¼×Ķ¬Ń§ĪŖĮĖČ·ČĻČÜŅŗ³ŹŗģŗÖÉ«µÄŌŅņŹĒÉś³ÉĮĖFe(OH)3£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
| ²½Öč | ²Ł×÷ | ĻÖĻó |
| ¢ņ | ÓĆ¼¤¹ā±ŹÕÕÉä²½Öč¢ńÖŠµÄŗģŗÖÉ«ČÜŅŗ | ³öĻÖ”°¶”“ļ¶ūŠ§Ó¦”± |
¼×Ķ¬Ń§Ņņ“ĖµĆ³ö½įĀŪ£ŗČÜŅŗ³ŹŗģŗÖÉ«ŹĒŅņĪŖÉś³ÉĮĖFe(OH)3”£¶ųŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µĆ³ö½įĀŪµÄÖ¤¾ŻČŌČ»²»×ć£¬ŅŅĶ¬Ń§µÄĄķÓÉŹĒ________________________________________________________________________”£
(4)ĪŖ½ųŅ»²½Č·ČĻNa2SO3ČÜŅŗÓėFeCl3ČÜŅŗ·“Ó¦µÄĒéæö£¬ŅŅĶ¬Ń§Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
| ²½Öč | ²Ł×÷ | ĻÖĻó |
| ¢ó | Ļņ1 mol·L£1µÄFeCl3ČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄSO2 | ČÜŅŗÓÉ»ĘÉ«±äĪŖŗģŗÖÉ« |
| ¢ō | ÓĆ¼¤¹ā±ŹÕÕÉä²½Öč¢óÖŠµÄŗģŗÖÉ«ČÜŅŗ | ƻӊ³öĻÖ”°¶”“ļ¶ūŠ§Ó¦”± |
¢Ł¾¼ģŃé²½Öč¢óÖŠŗģŗÖÉ«ČÜŅŗŗ¬ÓŠFe2£«£¬¼ģŃéFe2£«Ń”ÓƵďŌ¼ĮŹĒ________(Ģī×ÖÄø)”£
a£®K3[Fe(CN)6]ČÜŅŗ
b£®KSCNČÜŅŗ
c£®KMnO4ČÜŅŗ
¢ŚŅŃÖŖH2SO3ŹĒČõĖį£¬Ēė½įŗĻµēĄė·½³ĢŹ½ĖµĆ÷²½Öč¢óÖŠ³öĻÖŗģŗÖÉ«µÄŌŅņ£ŗ________________________________________________________________________”£
(5)½įĀŪ£ŗÓÉÉĻŹöŹµŃéµĆÖŖ£¬¼×”¢ŅŅĮ½Ķ¬Ń§Ėł³Ö¹Ūµć¾łÕżČ·”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŹµŃéŹŅ²śÉśµÄ·ĻŅŗÖŠŗ¬ÓŠFe3£«”¢Cu2£«”¢Ba2£«”¢Cl£ĖÄÖÖĄė×Ó£¬ĻÖÉč¼ĘĻĀĮŠ·½°ø¶Ō·ĻŅŗ½ųŠŠ“¦Ąķ£¬ŅŌ»ŲŹÕ½šŹō²¢ÖʱøĀČ»Æ±µ”¢ĀČ»ÆĢś¾§Ģ唣

(1)³Įµķ1ÖŠŗ¬ÓŠµÄ½šŹōµ„ÖŹŹĒ__________”£
(2)Ńõ»ÆŹ±¼ÓČėH2O2ČÜŅŗ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
________________________________________________________________________ӣ
(3)ĻĀĮŠĪļÖŹÖŠ£¬æÉŅŌ×÷ĪŖŹŌ¼ĮXµÄŹĒ__________(Ģī×ÖÄø)”£
A£®BaCl2 B£®BaCO3
C£®NaOH D£®Ba(OH)2
(4)¼ģŃé³Įµķ2Ļ“µÓŹĒ·ńĶźČ«µÄ·½·ØŹĒ__________”£
(5)ÖʱøĀČ»ÆĢś¾§Ģå¹ż³ĢÖŠŠč±£³ÖŃĪĖį¹żĮ棬ĘäÄæµÄŹĒ__________”£
(6)ÓɹżĀĖ2µĆµ½µÄĀĖŅŗÖʱøBaCl2µÄŹµŃé²Ł×÷ŅĄ“ĪĪŖ__________”¢ĄäČ“½į¾§”¢__________”¢Ļ“µÓ”¢øÉŌļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄÜĖµĆ÷BF3·Ö×ÓµÄ4øöŌ×ÓŌŚĶ¬Ņ»Ę½ĆęµÄĄķÓÉŹĒ(””””)
A£®Į½øö¼üÖ®¼äµÄ¼Š½ĒĪŖ120”ć
B£®B”ŖF¼üĪŖ·Ē¼«ŠŌ¹²¼Ū¼ü
C£®3øöB”ŖF¼üµÄ¼üÄÜĻąĶ¬
D£®3øöB”ŖF¼üµÄ¼ü³¤ĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ1 gĒāĘųĶźČ«Č¼ÉÕÉś³ÉĖ®ÕōĘųŹ±·Å³öČČĮæ 121 kJ£¬ĒŅŃõĘųÖŠ1 mol O===O¼üĶźČ«¶ĻĮŃŹ±ĪüŹÕČČĮæ496 kJ£¬Ė®ÕōĘųÖŠ1 mol H”ŖO¼üŠĪ³ÉŹ±·Å³öČČĮæ463 kJ£¬ŌņĒāĘųÖŠ1 mol H”ŖH¼ü¶ĻĮŃŹ±ĪüŹÕČČĮæĪŖ(””””)
A£®920 kJ B£®557 kJ
C£®436 kJ D£®188 kJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄæĒ°Į÷ŠŠµÄ¹ŲÓŚÉśĆüĘšŌ“¼ŁÉčµÄĄķĀŪČĻĪŖ£¬ÉśĆüĘšŌ“ÓŚŌ¼40ŅŚÄźĒ°µÄ¹ÅŃóµ×µÄČČŅŗ»·¾³£¬ÕāÖÖ»·¾³ĻµĶ³ÖŠĘÕ±é“ęŌŚĢśĮņ“Ų½į¹¹£¬ČēFe2S2”¢Fe4S4”¢Fe8S7µČ£¬ÕāŠ©ĢśĮņ“Ų½į¹¹²ĪÓėĮĖÉśĆüĘšŌ“µÄĻą¹Ų·“Ó¦”£Ä³»ÆѧŠĖȤŠ”×éŌŚŃŠ¾æijĢśĮņ“Ų½į¹¹µÄ×é³ÉŹ±£¬Éč¼ĘĮĖĻĀĮŠŹµŃ锣
”¾ŹµŃé¢ń”æ””Č·¶ØĮņµÄÖŹĮæ£ŗ
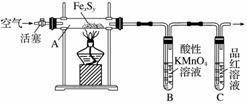
°“Ķ¼Į¬½Ó×°ÖĆ£¬¼ģ²éŗĆ×°ÖƵÄĘųĆÜŠŌŗó£¬ŌŚÓ²ÖŹ²£Į§¹ÜAÖŠ·ÅČė1.0 gĢśĮņ“Ų½į¹¹(ŗ¬ÓŠ²æ·Ö²»·“Ó¦µÄŌÓÖŹ)£¬ŌŚŹŌ¹Ü BÖŠ¼ÓČė50 mL 0.100 mol·L£1µÄĖįŠŌKMnO4ČÜŅŗ£¬ŌŚŹŌ¹ÜCÖŠ¼ÓČėĘ·ŗģČÜŅŗ”£ĶØČėæÕĘų²¢¼ÓČČ£¬·¢ĻÖ¹ĢĢåÖš½„×Ŗ±äĪŖŗģ×ŲÉ«”£“ż¹ĢĢåĶźČ«×Ŗ»Æŗ󣬽«BÖŠČÜŅŗ×ŖŅĘÖĮ 250 mL ČŻĮæĘ棬Ļ“µÓŹŌ¹ÜBŗó¶ØČŻ”£Č”25.00 mL øĆČÜŅŗÓĆ0.01 mol·L£1µÄ²ŻĖį(H2C2O4)ČÜŅŗµĪ¶ØŹ£ÓąµÄ KMnO4”£¼ĒĀ¼Źż¾ŻČēĻĀ£ŗ
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗĢå»ż/mL | ²ŻĖįČÜŅŗĢå»ż/mL | |
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
Ļą¹Ų·“Ó¦£ŗ¢Ł2MnO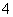 £«2H2O£«5SO2===2Mn2£«£«5SO
£«2H2O£«5SO2===2Mn2£«£«5SO £«4H£«
£«4H£«
¢Ś2MnO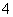 £«6H£«£«5H2C2O4===2Mn2£«£«10CO2”ü£«8H2O
£«6H£«£«5H2C2O4===2Mn2£«£«10CO2”ü£«8H2O
”¾ŹµŃé¢ņ”æ””Č·¶ØĢśµÄÖŹĮæ£ŗ
½«ŹµŃé¢ńÓ²ÖŹ²£Į§¹ÜAÖŠµÄ²ŠĮō¹ĢĢå¼ÓČėĻ”ŃĪĖįÖŠ£¬³ä·Ö½Į°čŗó¹żĀĖ£¬ŌŚĀĖŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬¹żĀĖŗóČ”ĀĖŌü£¬¾³ä·Ö×ĘÉÕµĆ0.6 g¹ĢĢ唣
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÅŠ¶ĻµĪ¶ØÖÕµćµÄ·½·ØŹĒ______________________________________________
________________________________________________________________________ӣ
(2)ŹŌ¹ÜCÖŠĘ·ŗģČÜŅŗµÄ×÷ÓĆŹĒ_____________________________________________”£
ÓŠĶ¬Ń§Ģį³ö£¬³·Č„C×°ÖƶŌŹµŃéƻӊӰĻģ£¬ÄćµÄæ“·ØŹĒ________(Ń”Ģī”°Ķ¬Ņā”±»ņ”°²»Ķ¬Ņā”±)£¬ĄķÓÉŹĒ___________________________________________________
________________________________________________________________________ӣ
(3)øł¾ŻŹµŃé¢ńŗĶŹµŃé¢ņÖŠµÄŹż¾ŻæÉČ·¶ØøĆĢśĮņ“Ų½į¹¹µÄ»ÆѧŹ½ĪŖ________________________________________________________________________”£
”¾ĪŹĢāĢ½¾æ”æ””µĪ¶Ø¹ż³ĢÖŠ£¬ĻøŠÄµÄĶ¬Ń§·¢ĻÖøĆKMnO4ČÜŅŗŃÕÉ«ĶŹČ„µÄĖŁĀŹ½ĻĘ½³£µĪ¶ØŹ±ŅŖæģµĆ¶ą”£ĪŖŃŠ¾æĖŁĀŹ¼ÓæģµÄŌŅņ£¬øĆĶ¬Ń§¼ĢŠų½ųŠŠĮĖĻĀĮŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
| ±ąŗÅ | ĪĀ¶Č/”ę | Ėį»ÆµÄH2C2O4ČÜŅŗ/mL | KMnO4ČÜŅŗ/mL | ČÜŅŗĶŹÉ«Ź±¼ä/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(Įķ¼ÓÉŁĮææÉČÜÓŚĖ®µÄMnSO4·ŪÄ©) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
(4)·ÖĪöÉĻŹöŹż¾Ż£¬µĪ¶Ø¹ż³ĢÖŠ·“Ó¦ĖŁĀŹ¼ÓæģµÄŅ»ÖÖæÉÄÜŌŅņŹĒ___________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼×°ÖĆÖŠXŗĶY¾łĪŖŹÆÄ«µē¼«£¬µē½āŅŗĪŖ500 mLijĄ¶É«ČÜŅŗ£¬µē½āŅ»¶ĪŹ±¼ä£¬¹Ū²ģµ½Xµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Yµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£»ČÜŅŗÖŠŌÓŠČÜÖŹĶźČ«µē½āŗó£¬Ķ£Ö¹µē½ā£¬Č”³öXµē¼«£¬Ļ“µÓ”¢øÉŌļ”¢³ĘĮ棬µē¼«ŌöÖŲ1.6 g”£ĻĀĮŠÓŠ¹ŲĖµ·ØÖŠ²»ÕżČ·µÄŹĒ(””””)
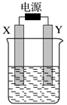
A£®Xµē¼«ŹĒŅõ¼«
B£®Yµē¼«²śÉśĘųĢåµÄĢå»żĪŖ0.28 L
C£®µē½āŗóČÜŅŗµÄpH£½1
D£®ŅŖŹ¹µē½āŗóČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬Šč¼ÓČėŅ»¶ØĮæµÄCuO»ņCuCO3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com