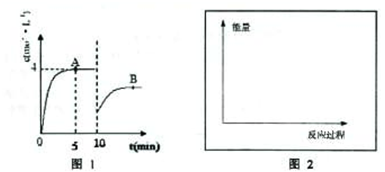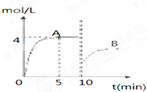
��һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L
-1��b mol?L
-1����ӦΪ��N
2+3H
2?2NH
3��������Ũ����ʱ��仯��ͼ��ʾ��
��1����Ӧ��5minʱ��������Ӧ����
1.2mol/��L?min��
1.2mol/��L?min��
��A��ƽ�ⳣ��Ϊ
���ú�a��b�ı���ʽ��ʾ��
��2����10minʱ��ȡ�Ĵ�ʩ��
�������ɵİ��������Ͱ�����Ũ��
�������ɵİ��������Ͱ�����Ũ��
��
��3��-50��Cʱ��Һ���������µ��룺2NH
3?NH
4++NH
-2��K=2��10
-12����Һ���м���NH
4Cl���壬K
=
=
2��10
-12�����������������=����
��4����֪2A
2��g��+B
2��g���T2C
3��g������H=-a kJ/mol��a��0������һ���д����Ĺ̶��ݻ��������м���2mol A
2��1mol B
2����500��ʱ��ַ�Ӧ��ƽ���C
3��Ũ��Ϊw mol/L���ų�����b kJ������ԭ���������У�ֻ����2mol C
3��500��ʱ��ַ�Ӧ��ƽ�����������c kJ��a��b��c֮��������ֹ�ϵ
b+c=a
b+c=a
���ô���ʽ��ʾ��

 ��һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L-1��b mol?L-1����ӦΪ��N2+3H2?2NH3��������Ũ����ʱ��仯��ͼ��ʾ��
��һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L-1��b mol?L-1����ӦΪ��N2+3H2?2NH3��������Ũ����ʱ��仯��ͼ��ʾ��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�