
����Ŀ�����õ�ζ����������һ�ִӻ���������ȡ�Ļӷ����㾫�ͣ��������Ҵ������ѵ��л��ܼ���������ͼ��ʾװ�ô��������ѷۣ��������ᴿ�õ������͡�
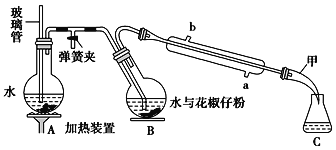
ʵ�鲽�裺
(һ)��Aװ���е�Բ����ƿ��װ��![]() �ݻ���ˮ���Ӽ�����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
�ݻ���ˮ���Ӽ�����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
(��)����Aװ���е�Բ����ƿ�����д�����������ʱ�رյ��ɼУ���������
(��)�����Һ�м���ʳ�������ͣ�����15mL������ȡ2�Σ���������ȡ���Ѳ�ϲ�������������ˮNa2SO4����Һ���㵹��������ƿ�У�����û����͡�
��1��װ��A�в����ܵ�������___��װ��B��Բ����ƿ��б��Ŀ����___��
��2������(��)�У����۲쵽������������ɫ��״Һ�����ʱ����ֹͣ�����������ʱ�����в�����˳��Ϊ___(����)��
��ֹͣ���� �ڴ��ɼ� �۹ر�����ˮ
��3�������Һ�м���ʳ�ε�������___��������ˮNa2SO4��������___��
��4��ʵ���������ϡNaOH��Һ��ϴ�����ܣ���Ӧ�Ļ�ѧ����ʽΪ___��
(�������� ��ʾ)
��ʾ)
��5��Ϊ�ⶨ����������֬�ĺ�����ȡ20.00mL�����������Ҵ��У���80.00mL0.5mol/LNaOH���Ҵ���Һ�����裬��ַ�Ӧ����ˮ���200mL��Һ��ȡ25.00mL�����̪����0.1moI/L������еζ����ζ��յ���������20.00mL����û������к�����֬___g/L��
(�� �ƣ�ʽ����884)��
�ƣ�ʽ����884)��
���𰸡�ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ���� ��ֹ�ɽ����Һ�������������(��������) �ڢ٢� ���ͻ�������ˮ�е��ܽ�ȣ������ڷֲ� ��ȥ�������е�ˮ����� 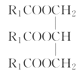 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+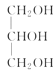 353.6
353.6
��������
��1��A�в�������������װ��A�в����ܵ�������ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ����װ��B��Բ����ƿ��б��Ŀ���Ƿ�ֹ�ɽ����Һ�������������(��������)���ʴ�Ϊ��ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ����ֹ�ɽ����Һ�������������(��������)��
��2������(��)�У������Ͳ�����ˮ�����۲쵽������������ɫ��״Һ�����ʱ����ֹͣ�����������ʱ�����ɼУ�ֹͣ���ȣ��ر�����ˮ��
�ʴ�Ϊ�� �ڢ٢ۣ�
��3�������Ͳ�����ˮ�������Һ�м���ʳ�ε������ǽ��ͻ�������ˮ�е��ܽ�ȣ������ڷֲ㣻������ˮNa2SO4�������dz�ȥ�������е�ˮ�����ʴ�Ϊ�����ͻ�������ˮ�е��ܽ�ȣ������ڷֲ㣻��ȥ�������е�ˮ����
��4��ʵ���������ϡNaOH��Һ��ϴ�����ܣ�������Ϊ���࣬��NaOH��Һ��ˮ��Ϊ�����ƺʹ�����Ӧ�Ļ�ѧ����ʽΪ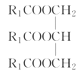 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+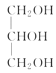 ��
��
�ʴ�Ϊ��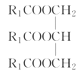 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+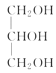 ��
��
��5������ζ�������NaOH���������n��NaOH��=n��HCl��=0.1mol��L��1��0.02L��![]() =0.016mol�������ˮ�ⷴӦ��n��NaOH��=0.5mol��L��1��0.08L=0.04mol-0.016mol=0.024mol���ʻ������е���֬���ʵ���Ϊ
=0.016mol�������ˮ�ⷴӦ��n��NaOH��=0.5mol��L��1��0.08L=0.04mol-0.016mol=0.024mol���ʻ������е���֬���ʵ���Ϊ![]() ��0.024mol=0.008mol����û������к�����֬Ϊ
��0.024mol=0.008mol����û������к�����֬Ϊ![]() =353.6g��L��1��
=353.6g��L��1��
�ʴ�Ϊ��353.6��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯���ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԣ�����˵����ȷ����
A. X��Y��Z��W��ԭ�Ӱ뾶���μ�С
B. W��X�γɵĻ�������ֻ�����Ӽ�
C. W����̬�⻯��ķе�һ������Z����̬�⻯��ķе�
D. ��W��Y��ԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ��������������ʵ���Ũ�ȵļ������������
A.�ܶ�Ϊ0.91g/cm3�İ�ˮ����������Ϊ25%���ð�ˮ�õ������ˮϡ�ͺ�������Һ�����ʵ�������������12.5%
B.��K2SO4��Al2(SO4)3�Ļ����Һ����֪����Al3�������ʵ���Ũ��Ϊ0.4 mol/L ��SO42�������ʵ���Ũ��Ϊ0.7 mol/L �������Һ��K�������ʵ���Ũ��Ϊ0.2 mol/L
C.��5 mol/L ��Mg(NO3)2��ҺamL ϡ����bmL��ϡ�ͺ���Һ��NO3�������ʵ���Ũ��Ϊ![]() mol/L
mol/L
D.����״���£���VL A���壨Ħ������ΪMg/mol������0.1Lˮ�У�������Һ�ܶȦ�g/cm3�������Һ�����ʵ���Ũ��Ϊ![]() mol/L
mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯������;�dz��㷺���ش��������⣺
��1�����ķ���ϩ��һ�����壬�þ�����һ����ƽ�����������ϸ�����λ����Ķ��ؾ��塣��ͨ��___�������־��塢����ͷǾ��塣
��2��KԪ�صĻ�̬ԭ�ӵĵ��������___����ͬ���ܼ���
��3��[H2F]+[SbF6]-(������)��һ�ֳ�ǿ�ᣬ����[H2F]+�������ӵĿռ乹��Ϊ___��д��һ����[H2F]+������ͬ�ռ乹�ͺͼ�����ʽ����������___��
��4��NH4F(�����)����Ϊ����ʴ�̼������������������ȡ�NH4+������ԭ�ӵ��ӻ�������___��������д���___(����ĸ)��
A.���Ӽ� B.���� C.���� D.���
��5��SbF6���㷺������ѹ�����豸��Ե���ʡ�SbF6��һ�ֹ��ۻ������ͨ������BornHaberѭ��������������ͼ(��ͼa)������ؼ��ܡ���F��F���ļ���Ϊ___kJ��mol-1��S��F�ļ���Ϊ___kJ��mol-1��
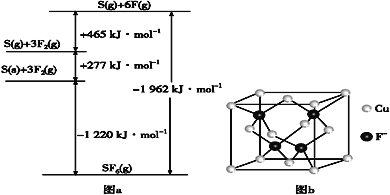
��6��CuCl���۵�Ϊ426�棬�ۻ�ʱ���������磻CuF���۵�Ϊ908�棬�ܶ�Ϊ7.1g��cm-3��
��CuF��CuCl�۵�ߵ�ԭ����___��
����֪NAΪ�����ӵ�������ֵ��CuF�ľ����ṹ����ͼb������CuF�ľ�������a=___nm(�г�����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼװ�ý���ʵ��̽������ش��������⣺

��1������ת��Ϊ��ѧ�ܵ�װ��Ϊ_______�أ�����A������B������
��2��Aװ��пΪ_______����ʵ�������������Ũ�Ƚϴ��������______������Zn�缫������Cu�缫������
��3��Bװ��ʯī2Ϊ_______�����缫��ӦʽΪ_______����ʯī1�ų�2240mL���壨��״���£�ʱ����·��ת�Ƶ��ӵ���ĿΪ_________����Aװ��Ҳת����ͬ�����ĵ��ӣ�п������������_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У�����ͼ����ʾ����ת����ϵ�Ҿ���һ������ʵ�ֵ���
ѡ�� | X | Y | Z |
A | Na | NaOH | Na2O2 |
B | Fe | FeCl2 | Fe(OH)3 |
C | NO | NO2 | HNO3 |
D | Al | Al2O3 | Al(OH)3 |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ�������������������϶�Ľ�����
(1)�����й�ͭԪ�ص�˵���У�����ȷ����__________������ĸ��
A.��ͭ������֡�Ӳ�����ǺϽ�
B.ͭ�������γ����ܵ�����Ĥ
C.ͭ��O2��Ӧ���ɺ�ɫ��CuO
D.CuSO4��5H2O��һ�ֻ������Ⱥ��Ϊ��ɫ����
(2)ij��ѧС��Ϊ�ⶨijͭ���������ͭ���������������������ʵ�鷽����
������ͭ�������![]() �ⶨ������������
�ⶨ������������
������ͭ�������![]() �ⶨʣ����������
�ⶨʣ����������
����д����������ͭ��ϡ���ᷴӦ�����ӷ���ʽ��___________���÷�����ϡ������ֳ�����������___________������ĸ����
A.�ӷ��� B.���� C.������ D.��ԭ��
�ڷ�����ȷ����������ʵʩ��������________________����д���������з�����Ӧ�Ļ�ѧ����ʽ��____________���÷�Ӧ���������ڱ�״���µ����Ϊ________(ͭ�������������m1g����Ӧ��ʣ����������m2g���ú�m1��m2�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ἰ������Һ��ɱ����������Ư�����á��ڴ˴ο����¹������з����˾����á�ijѧϰС�������Ҫ���Ʊ�Ũ�Ȳ�С��0.8mol��L-1�Ĵ�������Һ��
���������ϣ�
����1�����³�ѹ�£�Cl2O�ǻ���ɫ����ǿ�Ҵ̼�����ζ�����壬��һ��ǿ��������������ˮ���һ���ˮ��Ӧ���ɴ����ᡣ
����2��Cl2O�ķе�Ϊ3.8�棬42�����ϻ�ֽ�����Cl2��O2��Cl2�ķе�Ϊ-34.6��
��С����Cl2�볱ʪ��̼������ȡCl2O������һ����ȡ�����ᣬװ�����¡�
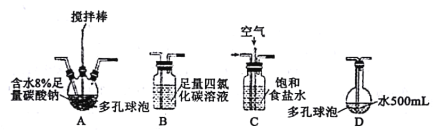
�ش��������⣺
��1��װ������˳��ΪCl2��___��������ĸ��ʾ��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽ��___��
��3��װ��B������___������ʵ��ӿ췴Ӧ���������õķ�����___��
��4��װ��D�з�Ӧ�Ļ�ѧ����ʽ��___��
��5�����Ҫ�ռ�Cl2O���������Bװ�ú�����Eװ�ý����ռ�����������������ʲô___���ݳ��������Ҫ�ɷ���___��
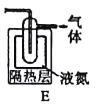
��6���˷��������������ֱ������ˮ�Ʊ���������Һ���ŵ���___�����һ�����ɣ���
��7���ⶨ��Ӧ��ɺ�A�����ʵ�����������ȡmg��Ʒ����������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1molL-1���������Һ�ζ�����Һ�ɺ�ɫ��Ϊ��ɫ����������V1mL�������ѱ���ɫ����Һ�м��뼸�μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ���ɫ������������V2mL��
����������ʵ��ɲⶨ��Ӧ��ɺ�A�����ʵ���������Ϊ___��˵���������ʲ��ú�m��V1��V2�Ĵ���ʽ��ʾ����
�����ü�����ָʾ���ζ�����ʱ���ζ��ܼ��������ݣ��Բⶨ�����Ӱ����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������ͼװ����ȡ�л������˵��������ǣ� ��

A.�����¶ȼƶ����IJ�ͬ������ȡ��ϩ������
B.bΪ��ѹ��Һ©�������ŵ��DZ���Һ��˳������
C.������������ͨ�����Ը��������Һ������Һ��ɫ����֤������ϩ����
D.Ũ�����ڷ�Ӧ�е����ÿ����У�������ˮ����������ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com