
��14�֣��ż�ͬѧ����Ư��Һ���Ʊ�ԭ���͵��ԭ��������һ�ּ��û���������Һ������(��ͼ��ʾ)����ʯī���缫��ⱥ���Ȼ�����Һ��ͨ��ʱ��ΪʹCl2����ȫ���գ��Ƶ��н�ǿɱ������������Һ�����Դ��bΪ____���������缫��ӦʽΪ�� __________________________������Ư��Һ�����ӷ���ʽΪ___________________________________________��
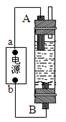
����ͬѧ�ܴ����������ô�װ����ȡFe(OH)2���������ҺΪ��������Һ��B�缫����Ϊʯī��A�缫����Ϊ_________���缫��ӦʽΪ��A��____________________��ͨ�����Һ�в�����ɫ�������ҽϳ�ʱ�䲻��ɫ��������������缫���ӵ�Դ��ͨ������ܹ۲쵽������������ܹ۲쵽������������_____________________________________________________������������Ļ�ѧ����ʽ��____________________________________________________��
��1���� 2Cl--2e-=Cl2�� Cl2+2OH-=Cl-+ClO-+H2O
��2��Fe Fe-2e-=Fe2+ ��ɫ��״����Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ
4Fe(OH)2+O2+2H2O=4Fe(OH)3
����������1������װ��ͼ��֪��A�缫�������������ռ�������A�缫����������a�ǵ�Դ�ĸ�����b�ǵ�Դ��������B�ǵ��ص���������Һ�е������ӷŵ磬�缫��Ӧʽ��2Cl--2e-=Cl2�������������������������ƣ��������ɵ�Ư��Һ�ķ���ʽ��Cl2+2OH-=Cl-+ClO-+H2O��
��2��Ҫ������������������A�缫����һ���������缫��Ӧʽ��Fe-2e-=Fe2+���缫���Ӻ���������Һ�е�OH���ŵ磬���������������������������ױ����������������� ��ɫ��״����Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ������ʽ��4Fe(OH)2+O2+2H2O=4Fe(OH)3��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������ij��ѧ����С���о�ŨH2SO4�������ԵĽ��۲�������ʵ����֤��
������ij��ѧ����С���о�ŨH2SO4�������ԵĽ��۲�������ʵ����֤���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����ѧ�߶�10�½���ϰ��ѧ�Ծ����������� ���ͣ������
��14�֣��ż�ͬѧ����Ư��Һ���Ʊ�ԭ���͵��ԭ��������һ�ּ��û���������Һ������(��ͼ��ʾ)����ʯī���缫��ⱥ���Ȼ�����Һ��ͨ��ʱ��ΪʹCl2����ȫ���գ��Ƶ��н�ǿɱ������������Һ�����Դ��bΪ____���������缫��ӦʽΪ�� __________________________������Ư��Һ�����ӷ���ʽΪ___________________________________________��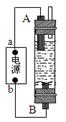
����ͬѧ�ܴ����������ô�װ����ȡFe(OH)2���������ҺΪ��������Һ��B�缫����Ϊʯī��A�缫����Ϊ_________���缫��ӦʽΪ��A��____________________��ͨ�����Һ�в�����ɫ�������ҽϳ�ʱ�䲻��ɫ��������������缫���ӵ�Դ��ͨ������ܹ۲쵽������������ܹ۲쵽������������_____________________________________________________������������Ļ�ѧ����ʽ��____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
������ͼ�ش����⣺
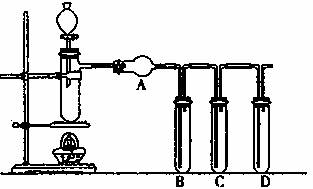
��1��װ�������Եļ�������ʢ��ҩƷǰ���С�������װ���У����Թ�B��C��D�и�����5 mL ����ˮ��ס���ܿڣ������Ӵ������ܶ���©��������£���ȼ�ƾ��ƣ��ȴ�֧�ܵ��Թ�һ������Թ�B��C��D��δ�������ݣ�Ϩ��ƾ��ƺ��Թ�B��C��D�е�����ˮ��Ҳδ��������ԭ���ǣ� ��
��2����һ����˵�������������������60 %���;���һ���������ԣ���ԽŨ������Խǿ�����¶�ҲҪӰ�������ԣ���ͭ�������Ũ�����з�Ӧ�����ԣ����ȾͿɹ۲쵽��������ͬѧ��������װ�ã���ʵ������֤���������Ե��������ۣ���֪98����Ũ������ܶ�Ϊ1.84g.m-3�����������Թ�B ����5mL Ʒ����Һ�����Թ�C��D �и�����5mL���ͳ���ʯ��ˮ�����֧�ܵ��Թ��з���ͭ�ۺ�3mLˮ��Ȼ��ӷ�Һ©����֧���Թ��еμ�98����Ũ����10�Σ�Լ0.5mL�������������ڣ���ʱ֧���Թ�����Һ��ɫ�Ƿ������Ա仯 ����ޡ����С������������ݽ�����ԭ�� ��
��ͬѧ�÷�Һ©������֧���Թ��м�98����Ũ����10mL�������������ڣ��۲쵽������ֱ��ǣ�֧���Թ��� ��B�Թ��� ��
��3����ͬѧ��������װ��������Ũ�����ľ̿���ڼ��������·�����Ӧ��ȫ������
��װ��A��Ӧ������Լ��� ��
��װ��B��C��D�������Լ��ֱ��� ������ĸ��ţ�
A������ʯ��ˮ��Ʒ����Һ���������������Һ
B��Ʒ����Һ���������������Һ������ʯ��ˮ
C���������������Һ��Ʒ����Һ������ʯ��ˮ
D������ʯ��ˮ���������������Һ��Ʒ����Һ
��ʵ��ʱΪ��ȷ��D����֤�Ľ�����ȷ��C��Ӧ�۲쵽�������� ��
��4����ͬѧ��Ϊ����������װ�������Cl2��Ư���ԣ������Ե�ʵ����֤������Ϊװ��A��B��C�����Լ�����Ʒ���п��ܵķֱ��� �� �� ������ͬѧ��Ϊ����װ����ɴ�ʵ�飬��û�Ӧ����һ��װ�ã�����Ϊ��װ�õ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����̨��2010������꼶����Բ��Ի�ѧ ���ͣ������
2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�
|
��ͬѧ��Ϊ�Ʊ�����Һ��̽����������һ�ּ��û���������
Һ��NaClO��Һ�����������������ͼ��ʾ��װ�ã���ʯī��
�缫��ⱥ���Ȼ�����Һ��������������⣺��ͨ��ʱ��Ϊ
ʹ���ɵ�Cl2����ȫ���գ��Ƶ��н�ǿɱ������������Һ��
���Դ��a�缫����Ϊ �������������������������
�������������������ĵ缫�ĵ缫��ӦʽΪ ��
װ����Һ�з�Ӧ����NaClO�����ӷ���ʽΪ
��
��ͬѧ����ij�����в�ѯ��ijƷ������Һ��װ˵���IJ�������ժ¼���£�
|
���������ʵ��̽�����̣�
I���Ķ����ϣ�����ѧ����֪ʶ�ж�����
��1�����������£�������Һ��NaClO����Һ��pH_____7�����������������������
ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________________ ��
��2��������Һ�����еĻ�ѧ������_________������ţ���
A��ǿ������ B��ǿ��ԭ��
C�����ȶ��� D��Ư����
E��������
II��ȷ��Ҫ�о�������
|
III����Ʒ�����ʵʩ̽��
��1�����ձ�ȡ������Ʒ����һ�Ź�������̼ͨ�ֶ������ձ�������һ��
ʱ�䡣Ԥ�ڵ�ʵ�������� ��
��2��Ϊ�˽�һ��̽��̼�ֶ��ڸ�����Һ��NaClO���еĸ�ʴԭ������ͬ
ѧ���������ͼʵ��װ�ã�д��̼��C�����Ϸ����ĵ缫��Ӧʽ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com