



 £¬·“Ó¦¢ÜĪŖ£ŗNH3+HClØTNH4Cl£¬ÓŠ°×ŃĢÉś³ÉµÄĻÖĻó£¬NH4Cl³£ÓĆ×÷ÖʵŖ·Ź£¬¹Ź“š°øĪŖ£ŗ
£¬·“Ó¦¢ÜĪŖ£ŗNH3+HClØTNH4Cl£¬ÓŠ°×ŃĢÉś³ÉµÄĻÖĻó£¬NH4Cl³£ÓĆ×÷ÖʵŖ·Ź£¬¹Ź“š°øĪŖ£ŗ £»°×ŃĢ£»ÖʵŖ·Ź£Ø»ņÖĘĻõĖį”¢ÖĘ“æ¼ī”¢ÖĘļ§ŃĪ£©µÄŌĮĻ»ņ×öÖĘĄä¼Į£»
£»°×ŃĢ£»ÖʵŖ·Ź£Ø»ņÖĘĻõĖį”¢ÖĘ“æ¼ī”¢ÖĘļ§ŃĪ£©µÄŌĮĻ»ņ×öÖĘĄä¼Į£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
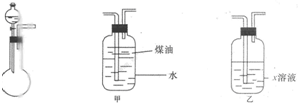 £Ø2010?“óĮ¬¶žÄ££©ÓŠŅ»»ÆѧѳʷNa2SO3£¬æÉÄÜŗ¬ÓŠNaCl”¢Na2SO4”¢KNO3”¢K2CO2”¢K2SO4ÖŠµÄŅ»ÖÖ»ņ¼øÖÖŌÓÖŹ£¬Ä³ŹµŃ銔×éĄūÓĆĶ¼Ģį¹©µÄ×°ÖĆČ·¶ØøĆѳʷµÄ³É·Ö¼°Na2SO3µÄÖŹĮæ·ÖŹż£®³Ę“Ėѳʷ6.30g£¬¼ÓČė6.0mol?L-1µÄĮņĖįÖĮ¹żĮ棬²śÉśĪŽÉ«ĘųĢå560mL£Ø±ź×¼×“æö£©£¬ĻņŅŻ³öĘųĢåŗóµÄČÜŅŗÖŠ¼ÓČėÉŌ¹żĮæµÄBaCl2ČÜŅŗ£¬µĆµ½°×É«³Įµķ9.32g£¬Ķø¹żĄ¶É«×ź²£Į§¹Ū²ģ£¬ĀĖŅŗµÄŃęÉ«·“Ó¦ĪŽ×ĻÉ«£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø2010?“óĮ¬¶žÄ££©ÓŠŅ»»ÆѧѳʷNa2SO3£¬æÉÄÜŗ¬ÓŠNaCl”¢Na2SO4”¢KNO3”¢K2CO2”¢K2SO4ÖŠµÄŅ»ÖÖ»ņ¼øÖÖŌÓÖŹ£¬Ä³ŹµŃ銔×éĄūÓĆĶ¼Ģį¹©µÄ×°ÖĆČ·¶ØøĆѳʷµÄ³É·Ö¼°Na2SO3µÄÖŹĮæ·ÖŹż£®³Ę“Ėѳʷ6.30g£¬¼ÓČė6.0mol?L-1µÄĮņĖįÖĮ¹żĮ棬²śÉśĪŽÉ«ĘųĢå560mL£Ø±ź×¼×“æö£©£¬ĻņŅŻ³öĘųĢåŗóµÄČÜŅŗÖŠ¼ÓČėÉŌ¹żĮæµÄBaCl2ČÜŅŗ£¬µĆµ½°×É«³Įµķ9.32g£¬Ķø¹żĄ¶É«×ź²£Į§¹Ū²ģ£¬ĀĖŅŗµÄŃęÉ«·“Ó¦ĪŽ×ĻÉ«£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
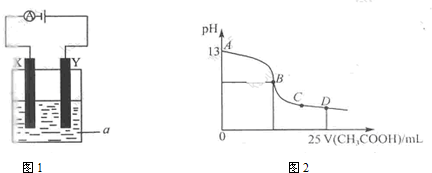
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
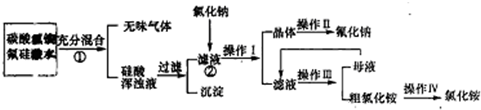
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com