
| 58.5b |
| c |
| 58.5b |
| c |
| 1000��b |
| ac-36.5b |
| 1000��b |
| ac-36.5b |
| m |
| M |
| m |
| �� |
| n |
| V |
| 1000bg��36.5% |
| 36.5 |
| 1 |
| 2 |
| 5bmol |
| c% |
| 500b |
| c |
| 500b |
| c |
| 1000b |
| c |
| 1000b |
| c |
| 58.5b |
| c |
| 58.5b |
| c |
| 1000b |
| c |
| 500b |
| c |
| 36.5b |
| c |
(a-
| ||
| ��kg/L |
| ac-36.5b |
| ��c |
| ||
|
| 1000��b |
| ac-36.5b |
| 1000��b |
| ac-36.5b |


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��̨��һģ����1��AΪ�ö��Ե缫��ⱥ��ʳ��ˮ��������Ca2+��Mg2+����װ�ã�
��2012?��̨��һģ����1��AΪ�ö��Ե缫��ⱥ��ʳ��ˮ��������Ca2+��Mg2+����װ�ã�| ���� | ��Ҫ���� | ���� | ���� |
| ����1�� | |||
| ����2��������H+��OH-�кͣ�������Һ��ɫ |
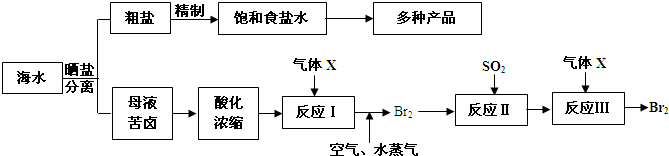
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ѧ2010��2011ѧ���һ��ѧ�����п��Ի�ѧ���� ���ͣ�022
| |||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ��ͨ��ͨ����ƽ������ѧ�߿���ѧģ���Ծ��������������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com