
ĻĀĶ¼ÖŠA~J·Ö±š“ś±ķÓŠ¹Ų·“Ó¦ÖŠµÄŅ»ÖÖĪļÖŹ£¬ĖüĆĒ¾łĪŖ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ”£ŅŃÖŖA~EŗĶF~JÖŠ·Ö±šŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ”£·“Ó¦E”śA+O2µÄĢõ¼žĪ“±ź³ö”£
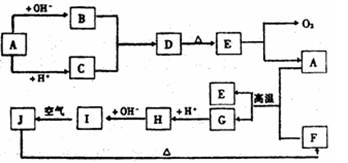
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©»ÆŗĻĪļHÖŠµÄŃōĄė×ÓŹĒ £»DµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©AÉś³ÉB·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø3£©AŗĶFµÄ»ģŗĻĪļ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©Š“³öBÓėCÉś³ÉDµÄĄė×Ó·½³ĢŹ½ ”£
£Ø5£©I”śJ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

ĒėĢīŠ“ŅŌĻĀæÕ°×£ŗ
(1)BµÄµē×ÓŹ½ĪŖ____________£¬D·Ö×ÓµÄæռ乹ŠĶĪŖ____________”£
(2)Š“³ö·“Ó¦¢Ł¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł___________________________________________£»
¢Ś___________________________________________”£
(3)·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø12·Ö£©ĻĀĶ¼ÖŠA~J·Ö±š“ś±ķĻą¹Ų·“Ó¦ÖŠµÄŅ»ÖÖĪļÖŹ£¬ŅŃÖŖA·Ö½āµĆµ½µČĪļÖŹµÄĮæµÄB”¢C”¢D£¬ŅŃÖŖB”¢DĪŖ³£ĪĀĻĀĘųĢ¬»ÆŗĻĪļ£¬CĪŖ³£ĪĀĻĀŅŗĢ¬»ÆŗĻĪļ£¬Ķ¼ÖŠÓŠ²æ·ÖÉś³ÉĪļĪ“±ź³ö”£
ĒėĢīŠ“ŅŌĻĀæÕ°×£ŗ
£Ø1£©AµÄ»ÆѧŹ½ £¬BµÄµē×ÓŹ½ £»
£Ø2£©H”¢I¶Ō»·¾³Ōģ³ÉµÄĪ£ŗ¦ÓŠ £»
£Ø3£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
D+ G”śH £»
F+J”śB +C +I ”£
£Ø4£©Š“³öA+NaOH”śDµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½Ī÷Ź”°×šŲ֎֊ѧøßŅ»µŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(10·Ö)ĻĀĶ¼ÖŠA~J·Ö±š“ś±ķÓŠ¹Ų·“Ó¦ÖŠµÄŅ»ÖÖĪļÖŹ£¬ĖüĆĒ¾łĪŖ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ”£ŅŃÖŖA~EŗĶF~JÖŠ·Ö±šŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ”£·“Ó¦E”śA+O2µÄĢõ¼žĪ“±ź³ö”£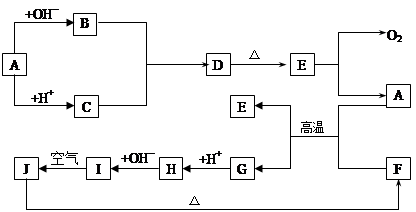
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©»ÆŗĻĪļHÖŠµÄŃōĄė×ÓŹĒ £»DµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©AÉś³ÉB·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø3£©AŗĶFµÄ»ģŗĻĪļ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©Š“³öBÓėCÉś³ÉDµÄĄė×Ó·½³ĢŹ½ ”£
£Ø5£©I”śJ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģ½Ī÷Ź”øßŅ»µŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(10·Ö)ĻĀĶ¼ÖŠA~J·Ö±š“ś±ķÓŠ¹Ų·“Ó¦ÖŠµÄŅ»ÖÖĪļÖŹ£¬ĖüĆĒ¾łĪŖ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ”£ŅŃÖŖA~EŗĶF~JÖŠ·Ö±šŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ”£·“Ó¦E”śA+O2µÄĢõ¼žĪ“±ź³ö”£
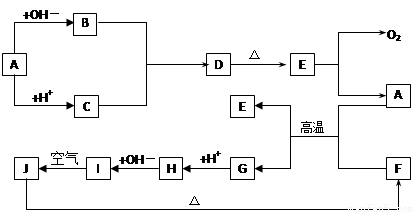
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©»ÆŗĻĪļHÖŠµÄŃōĄė×ÓŹĒ £»DµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©AÉś³ÉB·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø3£©AŗĶFµÄ»ģŗĻĪļ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©Š“³öBÓėCÉś³ÉDµÄĄė×Ó·½³ĢŹ½ ”£
£Ø5£©I”śJ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½ĖÕŹ”ĪŽĪżŹŠøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ĻĀĶ¼ÖŠA~J·Ö±š“ś±ķĻą¹Ų·“Ó¦ÖŠµÄŅ»ÖÖĪļÖŹ£¬ŅŃÖŖA·Ö½āµĆµ½µČĪļÖŹµÄĮæµÄB”¢C”¢D£¬ŅŃÖŖB”¢DĪŖ³£ĪĀĻĀĘųĢ¬»ÆŗĻĪļ£¬CĪŖ³£ĪĀĻĀŅŗĢ¬»ÆŗĻĪļ£¬Ķ¼ÖŠÓŠ²æ·ÖÉś³ÉĪļĪ“±ź³ö”£
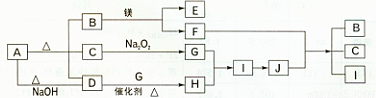
ĒėĢīŠ“ŅŌĻĀæÕ°×£ŗ
£Ø1£©AµÄ»ÆѧŹ½ £¬BµÄµē×ÓŹ½ £»
£Ø2£©H”¢I¶Ō»·¾³Ōģ³ÉµÄĪ£ŗ¦ÓŠ £»
£Ø3£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
D+ G”śH £»
F+J”śB +C +I ”£
£Ø4£©Š“³öA+NaOH”śDµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com