
����������Ϊ36.5%��Ũ����(�ܶ�Ϊ1.16 g��cm��3)���Ƴ�1 mol��L��1��ϡ���ᡣ��ʵ���ҽ���Ҫ��������220 mL���Իش��������⣺
(1)����ϡ����ʱ��Ӧѡ������Ϊ mL������ƿ��
(2)��������Ҫ mLŨ���ᣬ����ȡʱ��ѡ��������Ͳ�е� ��
| A��5 mL | B��10 mL | C��25 mL | D��50 mL |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��һ������NaOH��NaHCO3�Ļ����X�����ܱ������м��ȣ���ַ�Ӧ������V1L����Z(V1��0)����Ӧ��Ĺ������Y�������ϡ���ᷴӦ��������V2L����Z(V1��V2��Ϊ��״������������)�������жϲ���ȷ����
| A��V1>V2 | B��Z�ijɷ�ΪCO2 |
| C��Y�ijɷ�Ϊ Na2CO3 | D��X�� mol mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ʵ�����ù����ռ�����0��1 mol /L��NaOH��Һ500 mL���ش��������⣺
��1��������ҪNaOH��������� g��
��2����������������Ʒ�����ձ���ҩ�ע�250 mL����ƿ��500 mL����ƿ�ݲ�������������ƽ������ʱ������ʹ�õ���������Ʒ�� ������ţ�����ȱ�ٵ������� ��
��3��ʹ������ƿǰ������еĵ�һ�������� ��
��4������ʱ��һ���Ϊ���¼������裺�� ������ ����� �ܽ�� ҡ�Ȣ� ת�Ƣ� ϴ�Ӣ� ���ݣ�
����ȷ�IJ���˳��Ϊ ��
��5�����ƹ����У����в�����������ƫ�ߵ��� ������ţ���
�� δϴ���ձ��������� �� NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ������
�� ����ƿ�����������������ˮ �� ����NaOH��ʱ��̫�� �� ����ʱ���ӿ̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)(1) 0��5 mol SO2��Լ���� ��ԭ�ӣ����� gSO3������ԭ������ȡ�
��2��������ͬ�� ��HCl����NH3����CO2����O2���������У����з�����Ŀ���ٵ���(�����) _ ��
��3����100mL 0��2 mol/L ��NaOH��Һ��������Һϡ�͵�200 mL������Һ��Na+�����ʵ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ҫ�Ľ������ϣ�������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)�ǹ�ҵ����ȡ����ԭ�ϡ�ʵ����ģ�ҵ����������Ϊԭ����ȡAl2(SO4)3�����������[NH4Al(SO4)2��12H2O]�Ĺ���������ͼ��ʾ��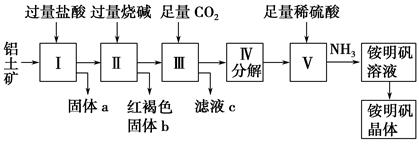
��ش��������⣺
(1)����a�Ļ�ѧʽΪ ������ͨ������CO2���巢����Ӧ�����ӷ���ʽΪ ��
(2)�ɢ���ȡ�������Һ�Ļ�ѧ����ʽΪ �����������Һ�л������������ʵ���������Ϊ(���������) ����ȴ�ᾧ������ϴ�ӡ�
(3)��1 000 kg��������36%��������Ϊԭ����ȡAl2(SO4)3����������������98%������(�ܶ�1.84 g��cm��1 L(����һλС��)��
(4)��ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1��1����Ͷ��ʱ�������е�Al2O3��H2SO4�����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��100 mL NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ�������̼�����V(��״����)��M������W�Ĺ�ϵ��ͼ
��ʾ����ش��������⣺
(1)b��ʱM����ɳɷ�Ϊ______________________��
(2)��Ҫʹb�����ɵ��ε�������Ϊ8.4 g����Ӧ��������Һ��ͨ�������̼________L(��״����)��
(3)�������ɵ�7.16 g�ε���Һ�м���һ������ij���ʣ���ַ�Ӧ��ѹ���������õ�������̼���ƹ���(�ᾧˮ)8.4 g��
����ֻ����0.03 molij���ʣ����������ʿ�����________��________��
����ֻ����0.06 molij���ʣ����������ʿ�����________��________��________��
(4)�����£�ͬŨ�ȵ�̼������Һ��̼��������Һ��pH
������7��������________��pH����������________________________��0.1 mol��L��1̼������Һ������Ũ�ȵĴ�С��ϵ��________����̼��������Һ����ε�������������Һ��������Ӧ�����ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������ֺ��϶࣬���ȵ�������ڷ����µ���Ա����Ҫ�Ĵ�ʩ�Ǹ���Ա������������ͼ��ҽԺ����Ա��Һʱ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ��ǩ��������۲��ǩ�����е����ݺ���д��
(1)�����ǵ�Ħ������Ϊ________��
(2)����Һ�к�ˮ________g��
(3)����Һ���ܶ�ԼΪ________g/mL��
(4)����Һ�����ʵ���Ũ��Ϊ________mol/L(��ȷ��С���������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ϳɰ���ҵ���������õĦ�Fe��������Ҫ�ɷ���FeO��Fe2O3��
��1��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4��5������Fe2����Fe3�����ʵ���֮��Ϊ________��
��2����������Fe2����Fe3�������ʵ���֮��Ϊ1��2ʱ�����������ߣ���ʱ����������������������������Ϊ________����С����ʾ������2λС������
��3����Fe2O3Ϊԭ���Ʊ������������������м�������̼�ۣ��������·�Ӧ��2Fe2O3��C 4FeO��CO2����
4FeO��CO2����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com