
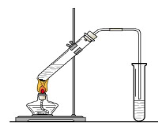
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
 ��
��

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢۢߢݢ� | B���ڢݢߢ� | C���٢ۢݢޢ� | D���ڢޢۢߢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ7.68g����ͭ������500mLˮ | B����ȡ12.0g�������500mL��Һ |
| C����ȡ8.0g����ͭ������500mLˮ | D����ȡ12.5g�������500mL��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��;�������û�����H2SO4��HNO3���ʵ���֮�����Ϊ3��2 |
| B�������;���١��ۣ�;���ڸ��õ���������ɫ��ѧ˼�� |
| C��1molCuSO4��1100�����û������X��O2һ��Ϊ0.75mol |
| D��Y������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ��
KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢܢݢޢߢ� | B���ڢۢݢޢߢ�� |
| C���٢ڢۢݢޢߢ� | D���٢ڢۢܢݢޢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com