
ŌŚ300 mLµÄĆܱÕČŻĘ÷ÖŠ£¬·ÅČėÄų·Ū²¢³äČėŅ»¶ØĮæµÄCOĘųĢ壬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗNi(s)£«4CO(g)Ni(CO)4(g)£¬ŅŃÖŖøĆ·“Ó¦µÄĘ½ŗā³£ŹżÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀ±ķ£ŗ
| ĪĀ¶Č/”ę | 25 | 80 | 230 |
| Ę½ŗā³£Źż | 5”Į104 | 2 | 1.9”Į10£5 |
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ (””””)”£
A£®ÉĻŹöÉś³ÉNi(CO)4µÄ·“Ó¦ĪŖ·ÅČČ·“Ó¦
B£®25 ”ꏱ·“Ó¦Ni(CO)4(g)Ni(s)£«4CO(g)µÄĘ½ŗā³£ŹżĪŖ2”Į10£5
C£®ŌŚ80 ”ꏱ£¬²āµĆijŹ±æĢNi(CO)4”¢COµÄÅØ¶Č¾łĪŖ0.5 mol”¤L£1£¬Ōņ“ĖŹ±
vÕż>vÄę
D£®80 ”ę“ļµ½Ę½ŗāŹ±£¬²āµĆn(CO)£½0.3 mol£¬ŌņNi(CO)4µÄĘ½ŗāÅضČĪŖ2 mol
”¤L£1
½āĪö””ĪĀ¶ČÉżøߣ¬Ę½ŗā³£Źż¼õŠ”£¬ĖµĆ÷Ę½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬Õż·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬AÕżČ·£»Ni(CO)4(g)Ni(s)£«4CO(g)ĪŖĢāøų·“Ó¦µÄÄę·“Ó¦£¬ĪĀ¶ČĻąĶ¬Ź±£¬Į½øö·“Ó¦µÄĘ½ŗā³£Źż»„ĪŖµ¹Źż¹ŲĻµ£¬BÕżČ·£»CĻīÖŠøĆŹ±æĢQc£½ £½8>K£¬·“Ó¦ÄęĻņ½ųŠŠ£¬vÄę>vÕż£¬C“ķĪó£»DĻīÖŠCOµÄĘ½ŗāÅضČĪŖ1 mol”¤L£1£¬ÓÉK£½2æɼĘĖć³öNi(CO)4µÄĘ½ŗāÅضČĪŖ2 mol”¤L£1£¬DÕżČ·”£
£½8>K£¬·“Ó¦ÄęĻņ½ųŠŠ£¬vÄę>vÕż£¬C“ķĪó£»DĻīÖŠCOµÄĘ½ŗāÅضČĪŖ1 mol”¤L£1£¬ÓÉK£½2æɼĘĖć³öNi(CO)4µÄĘ½ŗāÅضČĪŖ2 mol”¤L£1£¬DÕżČ·”£
“š°ø””C


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ė®ČČ·ØÖʱøFe3O4ÄÉĆ×æÅĮ£µÄ·“Ó¦ĪŖ3Fe2£«£«2S2O £«O2£«xOH£===Fe3O4£«S4O
£«O2£«xOH£===Fe3O4£«S4O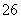 £«2H2O£¬ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ (””””)”£
£«2H2O£¬ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ (””””)”£
A£®ĆæÉś³É1 mol Fe3O4£¬·“Ó¦×ŖŅʵĵē×Ó×ÜŹżĪŖ4 mol
B£®Fe2£«ŗĶS2O ¶¼ŹĒ»¹Ō¼Į
¶¼ŹĒ»¹Ō¼Į
C£®1 mol Fe2£«±»Ńõ»ÆŹ±£¬±»Fe2£«»¹ŌµÄO2µÄĪļÖŹµÄĮæĪŖ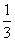 mol
mol
D£®x£½4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æĘѧ¼Ņ°ŃŅ©ĪļĮ¬ŌŚøß·Ö×ÓŌŲĢåEÉĻÖĘ³É»ŗŹĶ³¤Š§Ņ©¼Į”£°¢Ė¾Ę„ĮÖ
(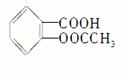 )æÉĮ¬½ÓŌŚÄ³øß·Ö×Ó¾ŪŗĻĪļÉĻ£¬ŠĪ³É»ŗŹĶ³¤Š§Ņ©¼Į£¬ĘäÖŠŅ»ÖÖ½į¹¹¼ņŹ½ĪŖ£ŗ
)æÉĮ¬½ÓŌŚÄ³øß·Ö×Ó¾ŪŗĻĪļÉĻ£¬ŠĪ³É»ŗŹĶ³¤Š§Ņ©¼Į£¬ĘäÖŠŅ»ÖÖ½į¹¹¼ņŹ½ĪŖ£ŗ
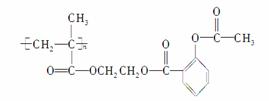
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŲĢå½į¹¹¼ņŹ½ĪŖ________________________________”£
(2)»ŗŹĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
________________________________________________________________________
________________________________________________________________________ӣ
(3)°¢Ė¾Ę„ĮÖŌŚ¼īŠŌĢõ¼žĻĀ(NaOH)·¢ÉśĖ®½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
________________________________________________________________________
________________________________________________________________________ӣ
(4)ÕāÖÖøß·Ö×ÓŌŲĢåŹĒÓɵ„Ģå·¢Éś¾ŪŗĻ·“Ó¦µĆµ½µÄ£¬Š“³öµ„ĢåµÄ½į¹¹¼ņŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢B”¢C”¢D¶¼ŹĒÖ»ŗ¬Ģ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲµÄÓŠ»śĪļ£¬ŌŚ³£ĪĀĻĀAĪŖĘųĢ¬£¬B”¢CĪŖŅŗĢ¬£¬DŹĒ°×É«¾§Ģ唣A”¢B¾łÓŠĒæĮŅ“Ģ¼¤ŠŌĘųĪ¶£¬CÓŠĻćĪ¶£¬DÓŠĢšĪ¶”£ĖüĆĒ¾ßÓŠĻąĶ¬µÄŹµŃéŹ½£¬·Ö±šŌŚŃõĘųÖŠ³ä·ÖČ¼ÉÕŗ󣬻Öø“µ½ŹŅĪĀĻĀ£¬ĘäČ¼ÉÕĖłĻūŗĵÄŃõĘųµÄĪļÖŹµÄĮæÓėČ¼ÉÕĖł²śÉśµÄĘųĢåµÄĪļÖŹµÄĮæĻąµČ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÉĻŹöÓŠ»śĪļµÄŹµŃéŹ½ŹĒ______________”£
(2)AµÄ½į¹¹¼ņŹ½ŹĒ____________£¬BµÄ½į¹¹¼ņŹ½ŹĒ____________£¬CµÄ½į¹¹¼ņŹ½ŹĒ______________£¬DµÄ½į¹¹¼ņŹ½ŹĒ____________»ņ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æÉÄę·“Ó¦A(g)£«2B(g)3C(g)£«4D(g)””¦¤H>0£¬ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ“ļµ½Ę½ŗāŗó£¬øıäijŅ»Ģõ¼ž£¬ĻĀĮŠĶ¼ĻńÕżČ·µÄŹĒ (””””)”£
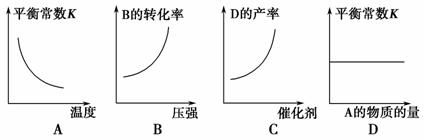
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČŻ»ż²»±äµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗ2SO2(g)£«O2(g)2SO3(g)””¦¤H<0”£ĻĀĮŠø÷Ķ¼±ķŹ¾µ±ĘäĖūĢõ¼ž²»±äŹ±£¬øıäijŅ»Ģõ¼ž¶ŌÉĻŹö·“Ó¦µÄÓ°Ļģ£¬ĘäÖŠ·ÖĪöÕżČ·µÄŹĒ (””””)”£
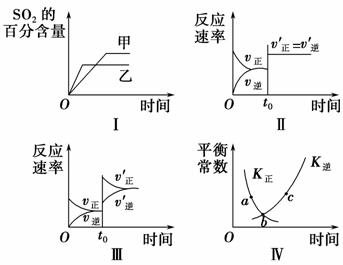
A£®Ķ¼¢ń±ķŹ¾ĪĀ¶Č¶Ō»ÆŃ§Ę½ŗāµÄÓ°Ļģ£¬ĒŅ¼×µÄĪĀ¶Č½Ļøß
B£®Ķ¼¢ņ±ķŹ¾t0Ź±æĢŹ¹ÓĆ“ß»Æ¼Į¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ
C£®Ķ¼¢ó±ķŹ¾t0Ź±æĢŌö“óO2µÄÅØ¶Č¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ
D£®Ķ¼¢ōÖŠa”¢b”¢cČżµćÖŠÖ»ÓŠbµćŅŃ¾“ļµ½»ÆŃ§Ę½ŗāדĢ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗĻ³É°±·“Ó¦ĪŖ£ŗN2(g)£«3H2(g)2NH3(g)”£Ķ¼1±ķŹ¾ŌŚŅ»¶ØµÄĪĀ¶ČĻĀ“Ė·“Ó¦¹ż³ĢÖŠµÄÄÜĮæµÄ±ä»Æ”£Ķ¼2±ķŹ¾ŌŚ2 LµÄĆܱÕČŻĘ÷ÖŠ·“Ó¦Ź±N2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆĒśĻß”£Ķ¼3±ķŹ¾ŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬øıäĘšŹ¼ĪļĒāĘųµÄĪļÖŹµÄĮæ¶Ō“Ė·“Ó¦Ę½ŗāµÄÓ°Ļģ”£

ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®øĆ·“Ó¦ĪŖ×Ō·¢·“Ó¦£¬ÓÉĶ¼1æÉµĆ¼ÓČėŹŹµ±µÄ“߻ƼĮ£¬EŗĶ¦¤H¶¼¼õŠ”
B£®Ķ¼2ÖŠ0”«10 minÄŚøĆ·“Ó¦µÄĘ½¾łĖŁĀŹv(H2)£½0.045 mol”¤L£1”¤min£1£¬“Ó11 minĘšĘäĖūĢõ¼ž²»±ä£¬Ń¹ĖõČŻĘ÷µÄĢå»żĪŖ1 L£¬Ōņn(N2)µÄ±ä»ÆĒśĻßĪŖd
C£®Ķ¼3ÖŠa”¢b”¢cČżµćĖł“¦µÄĘ½ŗāדĢ¬ÖŠ£¬·“Ó¦ĪļN2µÄ×Ŗ»ÆĀŹ×īøߵďĒbµć
D£®Ķ¼3ÖŠT1ŗĶT2±ķŹ¾ĪĀ¶Č£¬¶ŌÓ¦ĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖK1”¢K2£¬Ōņ£ŗT1>T2£¬K1>K2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°×Į×”¢ŗģĮ׏ĒĮ×µÄĮ½ÖÖĶ¬ĖŲŅģŠĪĢ壬ŌŚæÕĘųÖŠČ¼ÉÕµĆµ½Į×µÄŃõ»ÆĪļ£¬æÕĘų²»×揱ɜ³ÉP4O6£¬æÕĘų³ä×揱ɜ³ÉP4O10”£
(1)ŅŃÖŖ298 KŹ±°×Į×”¢ŗģĮ×ĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½·Ö±šĪŖP4(s£¬°×Į×)£«5O2(g)===P4O10(s) ¦¤H1£½£2 983.2 kJ”¤mol£1
P(s£¬ŗģĮ×)£«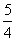 O2(g)===
O2(g)===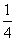 P4O10(s) ¦¤H2£½£738.5 kJ”¤mol£1
P4O10(s) ¦¤H2£½£738.5 kJ”¤mol£1
ŌņøĆĪĀ¶ČĻĀ°×Į××Ŗ»ÆĪŖŗģĮ×µÄČČ»Æѧ·½³ĢŹ½ĪŖ_______________________
_______________________________________________________________ӣ
(2)ŅŃÖŖ298 KŹ±°×Įײ»ĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖP4(s£¬°×Į×)£«3O2(g)===P4O6(s)
¦¤H£½£1 638 kJ”¤mol£1”£ŌŚÄ³ĆܱÕČŻĘ÷ÖŠ¼ÓČė62 g°×Į×ŗĶ50.4 LŃõĘų(±ź×¼×“æö)£¬æŲÖĘĢõ¼žŹ¹Ö®Ē”ŗĆĶźČ«·“Ó¦”£ŌņĖłµĆµ½µÄP4O10ÓėP4O6µÄĪļÖŹµÄĮæÖ®±ČĪŖ________£¬·“Ó¦¹ż³ĢÖŠ·Å³öµÄČČĮæĪŖ________”£
(3)ŅŃÖŖ°×Į×ŗĶPCl3µÄ·Ö×Ó½į¹¹ČēĶ¼ĖłŹ¾£¬ĻÖĢį¹©ŅŌĻĀ»Æѧ¼üµÄ¼üÄÜ(kJ”¤mol£1)£ŗP”ŖP 198£¬Cl”ŖCl 243£¬P”ŖCl 331”£Ōņ·“Ó¦P4(s£¬°×Į×)£«6Cl2(g)===4PCl3(s)µÄ·“Ó¦ČČ
¦¤H£½________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŅĶ¼ĪŖĪķö²µÄÖ÷ŅŖ³É·ÖŹ¾ŅāĶ¼”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
A£®ÖŲ½šŹōĄė×Óæɵ¼ÖĀµ°°×ÖŹ±äŠŌ
B£®±½ŹĒ×ī¼ņµ„µÄ·¼ĻćĢž
C£®SO2ŗĶNxOy¶¼ŹōÓŚĖįŠŌŃõ»ÆĪļ
D£®Ęū³µĪ²ĘųµÄ“óĮæÅÅ·ÅŹĒŌģ³ÉĪķö²ĢģĘųµÄČĖĪŖŅņĖŲÖ®Ņ»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com