
��֪ͬ�¶��µ��ܽ�ȣ�Zn(OH)2>ZnS��MgCO3>Mg(OH)2���ܽ������S2��������ΪFeS>H2S>CuS���������ӷ���ʽ�������(����)
A��Mg2����2HCO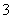 ��2Ca2����4OH��===Mg(OH)2����2CaCO3����2H2O
��2Ca2����4OH��===Mg(OH)2����2CaCO3����2H2O
B��Cu2����H2S===CuS����2H��
C��Zn2����S2����2H2O===Zn(OH)2����H2S��
D��FeS��2H��===Fe2����H2S��
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijǿ������ҺX�п��ܺ���Fe2+��A13+��NH4+��CO32����SO32����SO42����C1���е������֣���ȡX��Һ��������ʵ�飬ʵ����̼��������£�
����˵����ȷ���ǣ� ��
A��X�п϶�����Fe2+��NH4+��SO42�� B����ҺE������F���ܷ�����ѧ��Ӧ
C��X�п϶�������CO32����SO32����C1�� D������I��A1��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ�����У���д��˵����ȷ����
(����)��
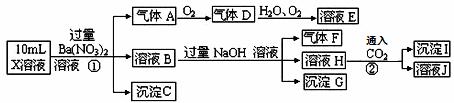
A���������Ľṹ��ʽ��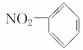
B���Ҵ����еĹ������ǡ�OH����һ����λ�����
C����������ģ��Ϊ
D������ķ���ʽ��C2H4O
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ��������ͼ��ʾ��װ����ȡ����������
ʵ��������ͼ��ʾ��װ����ȡ����������
(1)���Թ�������һ���������Ҵ��������Ũ����Ļ����Һ���䷽����______________��
(2)װ����ͨ�����ĵ���Ӧ���ڱ���̼������Һ��Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ��������ɵ�����ԭ����_________________��
(3)Ũ����������Ǣ�________����________��
(4)����̼������Һ��������______________________��
(5)��Ӧʱ���ɵ����������ܶȱ�ˮ________����________��ζ��
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ�������c(OH��)��1��10��13 mol��L��1����Һ�У��ܴ����������������(����)
A��Fe2����Na����NO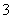 ��Cl��
��Cl��
B��Ba2����Na����NO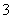 ��Cl��
��Cl��
C��SO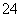 ��SO
��SO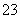 ��NH
��NH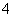 ��Na��
��Na��
D��Mg2����Na����Br����ClO��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.10 mol��L��1 KOH��Һ�ζ�10.00 mL 0.10 mol��L��1 H2C2O4(��Ԫ����)��Һ���õζ�������ͼ(�����Һ������ɿ��ɻ��ǰ��Һ�����֮��)����ش��������⣺
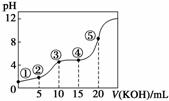 (1)�����ʾ��Һ�У�Kw��__________��
(1)�����ʾ��Һ�У�Kw��__________��
(2)�����ʾ��Һ�еĵ���غ�ʽΪ_________________��
(3)�����ʾ��Һ�д���________��ƽ�⡣
(4)�����ʾ��Һ�е������غ�ʽΪ0.10 mol��L��1��
________________________________________________________________________��
(5)�����ʾ��Һ�и�����Ũ�ȵĴ�С˳��Ϊ____________________________________��
(6)����5����ʾ��Һ�У�ˮ�ĵ���̶�������______����С����________(����Żش�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����벻����ѧ�����ж������и������ʵ���ʶ����ȷ����(����)
A����ͨ��������Ҫ�ɷ��Ǹ�֬��������
B����Ƥ���̷ۡ����д����ж����ʣ�����ʳ��
C��մ��Ѫ��������Ҫ����ˮ�ͼ�øϴ�·۽���
D�������⻯��Ӧ��Һֲ̬����ת��Ϊ��̬��ʽ֬����Ĺ��̷������ǻ�ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ѧ�ҷ���ijҩ��M������Ѫ�ܼ���������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��R��OH��HO��NO2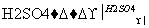
R��O��NO2��H2O(R��������)
��Ӧ�ڵĻ�ѧ����ʽΪ____________________________________________________
________________________________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������______ g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ΪʹF e 2+, F e 3+, Z n 2+����ȫ���γ������������, ��Һ�����ȷֱ�ΪpH 7��7�� pH 4��5�� pH 6��6��ij����п������Һ�к�������F e 2+, F e 3+��������, Ϊ��ȥ��Щ�����Ƶô�����Z n S O4, Ӧ������Լ��� �� ��
A��N a OH��Һ B����ˮ C��KMn O4, Z n CO3 D��H2O2, Z n O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com