
 ��
��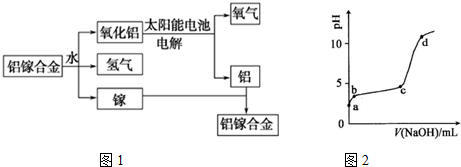
���� ��1����ԭ�Ӻ�����13�����ӣ��������������Ӳ㣬�����3�����ӣ�����������ԭ��ʧȥ���������γɣ�
��2�����غϽ���ˮ��Ӧ�õ����������������أ��൱����δ�μӷ�Ӧ��������ˮ��Ӧ��������ԭ��Ӧ����������ͨ�������µ��������������������������̫�����ṩ����ʼ�ķ�Ӧ�������غϽ����յ������������غϽ�
�����̷�����֪�غϽ���ˮ�ķ�Ӧ�������������������������أ��൱����δ�μӷ�Ӧ������ʵ���Ϸ����ķ�Ӧ������ˮ��Ӧ��������������������
�ڸù�����������ת����ʽ�У�̫���ܵ�ص����������̫����ת��Ϊ���ܡ�����ת��Ϊ��ѧ�ܣ�����ˮ��Ӧ��������ԭ��Ӧ����Ӧ�����з����ǻ�ѧ��ת��Ϊ���ܣ�������������ʽ������ת����
�۸ù����з����ķ�Ӧ�У�2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2���٣�2Al2O3$\frac{\underline{\;���\;}}{\;}$4Al+3O2�ڣ�������ʽ�١�2+�ڵ÷���ʽ2H2O$\frac{\underline{\;һ������\;}}{\;}$H2��+O2����
��3�������£���0.2mol/LAl2 �� SO4��3��Һ����μ���1.0mol/L NaOH��Һ��������Ϊǿ�������Σ�ˮ�������ԣ�ˮ�����ӷ�ӦΪAl3++3H2O?Al��OH��3+3H+a-b�Σ�����NaOH��Һ�����������ӣ�������Ӧ��H++OH-�TH2O��b-c����Һ��pH�仯������Ҫ������Ӧ��Al3++3OH-�TAl��OH��3����������OH-��Ҫ��������Al��OH��3������c-d����ҺpH�仯�ϴ�c���pH����ͻ�䣬NaOH������Al��OH��3������ʼ�ܽ⣬����NaAlO2�����Խ�ǿ��
��� �⣺��1����ԭ�Ӻ�����13�����ӣ��������������Ӳ㣬�����3�����ӣ�����������ԭ��ʧȥ���������γɣ����ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��2�������غϽ���ˮ�ķ�Ӧ�������������������������أ��൱����δ�μӷ�Ӧ������ʵ���Ϸ����ķ�Ӧ��2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2����
�ʴ�Ϊ��2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2����
������ͼ�з�����֪���ù�����������ת����ʽ�У�̫����ת��Ϊ���ܣ�2Al2O3$\frac{\underline{\;���\;}}{\;}$4Al+3O2��������ת��Ϊ��ѧ�ܣ�2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2������ѧ��ת��Ϊ���ܣ�������������ʽ������ת����
�ʴ�Ϊ��̫����ת��Ϊ���ܣ�����ת��Ϊ��ѧ�ܣ���ѧ��ת��Ϊ���ܣ�
�۸ù����з����ķ�Ӧ�У�2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2���٣�2Al2O3$\frac{\underline{\;���\;}}{\;}$4Al+3O2�ڣ�������ʽ�١�2+�ڵ÷���ʽ2H2O$\frac{\underline{\;һ������\;}}{\;}$H2��+O2�������Է�Ӧ��ʵ����ˮ�ķֽⷴӦ����ѧ����ʽΪ��2H2O$\frac{\underline{\;һ������\;}}{\;}$2H2��+O2����
�ʴ�Ϊ��2H2O$\frac{\underline{\;һ������\;}}{\;}$2H2��+O2����
��3��������Ϊǿ�������Σ�ˮ�������ԣ�ˮ�����ӷ�ӦΪAl3++3H2O?Al��OH��3+3H+��a-b�Σ�����NaOH��Һ�����������ӣ�������Ӧ��H++OH-�TH2O��b-c����Һ��pH�仯������Ҫ������Ӧ��Al3++3OH-�TAl��OH��3����������OH-��Ҫ��������Al��OH��3������c-d����ҺpH�仯�ϴ�c���pH����ͻ�䣬NaOH������Al��OH��3������ʼ�ܽ⣬����NaAlO2����ӦΪ��Al��OH��3+NaOH�TNaAlO2+2H2O����ϵ��Al��OH��3��NaAlO2�Ļ�ϣ�����NaAlO2Ũ�ȵ�������Խ�ǿ��pH����
�ʴ�Ϊ��Al��OH��3+NaOH�TNaAlO2+2H2O��c�㿪ʼ��Ҫ��Al��OH��3����NaOH�������Լ��Ե�NaAlO2����ϵ��Al��OH��3��NaAlO2�Ļ�ϣ�����NaAlO2Ũ�ȵ�����pH���ù�������������кͣ����pH��ͻԾ��
���� ���⿼����ԭ�ӽṹ�����̷���������ˮ�⡢ͼ������жϣ�ע���������������Լ�ͼ��c��pHͻ��Ϊ���Ĺؼ�����ȷb��c���������ӹ����������е������仯����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֬�ᣨC15H31COOH�� | B�� | ���ᣨC17H33COOH�� | ||
| C�� | ���ᣨHOOC-COOH�� | D�� | �����ᣨH2N-CH2COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ƵĻ�ѧʽ��Ca2O2 | |
| B�� | 1mol�������ƻ�������Ƹ�����ˮ��Ӧ������0.5mol���� | |
| C�� | ��������������������֮��Ϊ2��1 | |
| D�� | ����������ֻ�����Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | ���볣��K1 | ���볣��K2 |
| H2SO3 | 1.54��10-2 | 1.02��10-7 |
| H2CO3 | 4.3��10-7 | 5.6��10-11 |
| A�� | pH��Na2CO3��ҺС��Na2SO3��Һ | |
| B�� | ���H+������CO32-����SO32- | |
| C�� | NaHSO3��Һ�����Ե�ԭ���ǣ�NaHSO3�TNa++H++SO32- | |
| D�� | �������Һ�������Ϻ����Һ�У�c��SO32-����c��CO32-����c��HCO3-����c��HSO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3 | B�� | KCl | C�� | CaCl2 | D�� | LiCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��Ԫ��ֻ������ | |
| B�� | ����ֲ��������̵�������Ȼ�̵� | |
| C�� | ����Ԫ��Ҳ�����˵�ѭ�� | |
| D�� | ��������ͺ����л�����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ʯī�缫���200mL CuSO4��Һ���������е���ת�����ʵ���n��e-��������������V ��g������״�����Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ���ǣ�������
��ʯī�缫���200mL CuSO4��Һ���������е���ת�����ʵ���n��e-��������������V ��g������״�����Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ���ǣ�������| A�� | ���ǰCuSO4��Һ�����ʵ���Ũ��Ϊ2mol/L | |
| B�� | ����������Һ��c��H+��=2mol/L | |
| C�� | ��n��e-��=0.6molʱ��V��H2����V��O2��=2��3 | |
| D�� | ��������Һ�м���16gCuO������Һ�ɻָ�Ϊ���ǰ��Ũ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com