
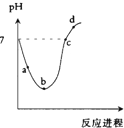
 ���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1mol/L��NaOH��Һ��������������Һ��pH�仯������ͼ��ʾ������ѡ����ȷ���ǣ�������
���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1mol/L��NaOH��Һ��������������Һ��pH�仯������ͼ��ʾ������ѡ����ȷ���ǣ�������| A��a����ʾ����Һ��c��H+��=c��Cl-��+c��HCl0��+c��OH-�� | B��b����ʾ����Һ��c��H+����c��Cl-����c��HClO����c��ClO-�� | C��c����ʾ����Һ��c��Na+��=c��HCl0��+c��ClO-�� | D��d����ʾ����Һ��c��Na+����c��ClO-����c��Cl-����c��HClO�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
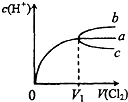 ��2010?��ƽ��һģ��һ���¶��£���Cl2����ͨ��ˮ�У���ͨ���Cl2���ΪV1ʱ�ﵽ���ͣ���Һ��c��H+���仯��ͼ������a����֪Cl2���ܽ�����¶����߶�Ѹ�ٽ��ͣ�������������ȷ���ǣ�������
��2010?��ƽ��һģ��һ���¶��£���Cl2����ͨ��ˮ�У���ͨ���Cl2���ΪV1ʱ�ﵽ���ͣ���Һ��c��H+���仯��ͼ������a����֪Cl2���ܽ�����¶����߶�Ѹ�ٽ��ͣ�������������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����5��ģ����������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1mol/L��NaOH��Һ,���������� ��Һ��pH�仯������ͼ��ʾ������ѡ����ȷ����

A. a����ʾ����Һ��
B. b����ʾ����Һ��
C. c����ʾ����Һ��
D. d����ʾ����Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1mol/L��NaOH��Һ,���������� ��Һ��pH�仯������ͼ��ʾ������ѡ����ȷ����
A. a����ʾ����Һ�� C(H+)=c(Cl-)+c(HClO)+c(OH-)
B. b����ʾ����Һ�� C(H+)��c(Cl-)�� c(HClO) �� c(ClO-)
C. c����ʾ����Һ�� C(Na+)=c(HClO) + c(ClO-)
D. d����ʾ����Һ�� C(Na+)��c(ClO-)�� c(Cl-)��c(HClO)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����ʮ���и���5��ģ����������ۺϻ�ѧ�Ծ����������� ���ͣ���ѡ��
���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1mol/L��NaOH��Һ,������������Һ��pH�仯������ͼ��ʾ������ѡ����ȷ����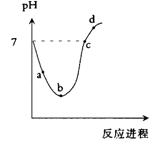
A��a����ʾ����Һ�� |
B��b����ʾ����Һ�� |
C��c����ʾ����Һ�� |
D��d����ʾ����Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com