
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����| A-B | A=B | A��B | ||
| CO | ���ܣ�kJ?mol-1�� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵ��kJ?mol-1�� | 441.2 273 | |||
| N2 | ���ܣ�kJ?mol-1�� | 154.8 | 418.3 | 941.7 |
| ���ܲ�ֵ��kJ?mol-1�� | 263.6 523.3 | |||
 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?���ݶ�ģ����Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼ��������Դ��Ѱ�÷�չ���¶��������͵���ڹ�ҵ�ͺ��պ���ҵ�ϱ��㷺����ʹ�ã�
��2011?���ݶ�ģ����Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼ��������Դ��Ѱ�÷�չ���¶��������͵���ڹ�ҵ�ͺ��պ���ҵ�ϱ��㷺����ʹ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�걱���߿�ģ��ϵ���Ծ�һ���۲��֣��������� ���ͣ������
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����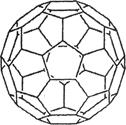
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵ���Χ�����Ų�ʽ__________����λ�����ڱ�____________����
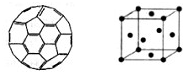
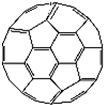
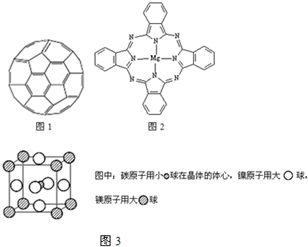
��2������ϩ���������ھ������õĹ�����ܣ���̫���ܵ�ص�Ӧ���Ͼ��зdz�������ǰ;������ϩ��C60���Ľṹ����ͼ��������̼ԭ�ӹ�����ӻ�����Ϊ________��1 mol C60�����ЦҼ�����ĿΪ____________����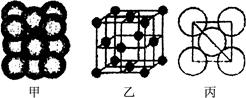
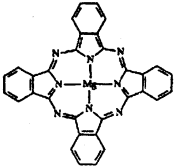
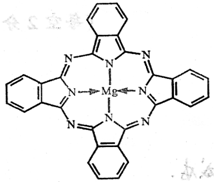
��3�� Cu���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��������Cuԭ�ӵ���λ��Ϊ____________��һ��������Cuԭ�ӵ���ĿΪ________��
��4�� Fe��CO��5�����³�Һ̬���۵�Ϊ��20��5 �棬�е�Ϊ103 �棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������________��������ͣ���Fe��CO��5���������壮��λ���ֱ���________��________��
��5������˵����ȷ����________��
| A����һ�����ܴ�С��S��P��Si |
| B���縺��˳��C��N��O��F |
| C����Ϊ������CaO��KCl�ߣ�����KCl��CaO�۵�� |
| D��SO2��CO2�Ļ�ѧ�������ƣ����ӽṹҲ����ֱ���ͣ���ͬ������SO2���ܽ�ȸ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com