
������N2H4��������������һ����Ҫ�Ļ��ȼ�ϡ�N2H4��N2O4��Ӧ�ܷų��������ȡ�
��1����֪��2NO2(g)  N2O4(g) ��H=-57.20 kJ��mol-1��һ�������£������һ�����ܱ������з�Ӧ2NO2(g)
N2O4(g) ��H=-57.20 kJ��mol-1��һ�������£������һ�����ܱ������з�Ӧ2NO2(g) N2O4(g)�ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ��� (����ĸ����
N2O4(g)�ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ��� (����ĸ����
A����СNO2��Ũ�� B�������¶� C������NO2��Ũ�� D�������¶�
��2��25��ʱ��1.00 g N2H4(l)������N2O4(l)��ȫ��Ӧ����N2(g)��H2O(l)���ų�19.14 kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3��17�桢1.01��105 Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c(NO2)=0.0300 mol��L-1��c(N2O4)=0.0120 mol��L-1����Ӧ2NO2(g) N2O4(g)��ƽ�ⳣ��K=_________
N2O4(g)��ƽ�ⳣ��K=_________
��4������һ������Cu��������ŨHNO3��Ӧ���Ƶ�1.00 L�Ѵﵽƽ���N2O4��NO2�Ļ�����壨17�桢1.01��105 Pa��������������������Cu________ g
��1����4�֣�B C
��2����3�֣�2N2H4(l)+N2O4(l)=3N2(g)+4H2O(l)�� ��H=-1224.96 kJ��mol-1
��3����3�֣�13.3
������֪ƽ��ʱ��c��N2O4��=0.0120 mol��L-1��c(NO2)=0.0300 mol��L-1
��4����4�֣�
�ɣ�3����֪����17�桢1.01��105Pa�ﵽƽ��ʱ��1.00 L��������У�
n(N2O4)= c(N2O4)��V=0.0120 mol��L-1��1.00L=0.0120mol
n(NO2)= c(NO2)��V=0.0300 mol��L-1��1.00L=0.0300mol
�� n (NO2) ��= n(NO2)��2n(N2O4)=0.0540mol
��Cu��4HNO3=Cu(NO3)2��2NO2����2H2O�ɵ�
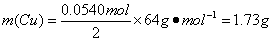


 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� �� ��
A��H2SO4 ��Ħ�������� 98 g B��1 mol H2O �������� 18 g/mol
C��Cl-��Ħ�������� 35.5 g/mol D��1 mol N2 ������� 22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£���ӦN2(g)��O2(g)==2NO(g)���ܱ������н��У����д�ʩ���ı仯ѧ��Ӧ���ʵ���
A��������ϵ�¶� B�����ݣ�����N2
C�����ݣ�����He D����ѹ������He
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ij��ѧʵ��С��Ϊȷ����������ֽ����Ѵ�����������ͼװ�ý���ʵ�飬��Ӧ�������ͷ�Ӧֹͣ��ʱ���������±���
|
ʱ�� H2O2 | 0.1 g | 0.3 g | 0.8 g |
| 10 mL 1.5% | 223 s | 67 s | 56 s |
| 10 mL 3.0% | 308 s | 109 s | 98 s |
| 10 mL 4.5% | 395 s | 149 s | 116 s |

��ش��������⣺
��1��ʢװ˫��ˮ�Ļ�ѧ���������� ��
��2����μ������װ�õ������� ��
��3����ͬŨ�ȵĹ������⣬��ֽ��������Ŷ����������������Ӷ�________��
��4����ʵ��Ч���͡���ɫ��ѧ���ĽǶȿ��ǣ�˫��ˮ��Ũ����ͬʱ������________g�Ķ�������Ϊ�ϼ�ѡ��
��5��ijͬѧ�����������ݺ���Ϊ����������ͬ�����Ķ�������ʱ��˫��ˮ��Ũ��ԽС������Ҫ��ʱ���Խ�٣��༴�䷴Ӧ����Խ�족�Ľ��ۣ�����Ϊ�Ƿ���ȷ________��������_______________________________________��(��ʾ��H2O2���ܶȿ���Ϊ�������)��
 ��������ͼ��ʾ�����ȼ��У���100mL 0.50mol��L-1CH3COOH��
��������ͼ��ʾ�����ȼ��У���100mL 0.50mol��L-1CH3COOH��
Һ��100mL0.55mol��L-1NaOH ��Һ��ϣ��¶ȴ�25.0�����ߵ�27.7�档��֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1���������������
150.5J����-1������Һ�ı�����Ϊ4.184J��g-1����-1����Һ���ܶȾ�����
Ϊ1g��mL-1��
��1������CH3COOH���к��ȡ�H= ��
��2��CH3COOH���к��ȵ�����ֵΪ56.1KJ�� mol-1���������
���ڣ�1���в�õ�ʵ��ֵƫ����ܵ�ԭ�� ��
��3��ʵ����NaOH������Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������Ӧ�Ľ�����ȷ����
 |
A B C D
| ʵ�� | ���� | ���� |
| A | ��ƿ��������������ձ���Һ������ | Cl��C��Si�ǽ��������μ��� |
| B | �ұ��Թ���ð�������ݿ� | FeCl3�ܼӿ�H2O2�ֽ����� |
| C | �Թ��г�ɫ��Һ��Ϊ��ɫ | �Ҵ�������ȥ��Ӧ������ϩ |
| D | �Թ�����Һ���γ��ְ�ɫ������ɫ����ɫ���� | �ܽ�ȣ�AgCl��AgBr��Ag2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и��������У�Y������X��Ӧ������Z��Ӧ����(����)
| X | Y | Z | |
| �� | NaOH��Һ | Al(OH)3 | ϡ���� |
| �� | KOH��Һ | SiO2 | Ũ���� |
| �� | O2 | N2 | H2 |
| �� | FeCl3��Һ | Cu | Ũ���� |
A.�٢ۡ�����B���٢ܡ�����C���ڢܡ�����D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̽�����Ʊ�����ˮ�ɷֵ�ʵ���У����и���ʵ������ó��Ľ��۲���ȷ����(����)
A����ˮ����ɫ��dz����ɫ��˵����ˮ�к���Cl2
B������ˮ�еμ������ữ��AgNO3��Һ��������ɫ������˵����ˮ�к���Cl��
C������ˮ�м���NaHCO3��ĩ�������ݲ�����˵����ˮ�к���H��
D����FeCl2��Һ�еμ���ˮ����Һ��ɫ����ػ�ɫ��˵����ˮ�к���HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��
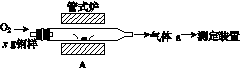
������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2 1________��3________��
1________��3________��
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����

��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����

������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com