
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1���嶡��ķ�Ӧ���£�
NaBr + H2SO4==== HBr + NaHSO4 ��
R��OH + HBrR��Br + H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br����Ũ��������ΪBr2�ȡ��й������б����£�
|
| �Ҵ� | ������ | ������ | 1���嶡�� |
| �ܶ�/g��cm��3 | 0��7893 | 1��4604 | 0��8098 | 1��2758 |
| �е�/�� | 78��5 | 38��4 | 117��2 | 101��6 |
��ش��������⣺
��1�� �������ˮ���� ������ڡ��������ڡ���С�ڡ�������
��2����1���嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã������� ������� �㡱�����²㡱���ֲ㡱��
��3���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ���� ��������ĸ��
����a�����ٸ�����ϩ���ѵ����� b������Br2������
����c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��4������ȥ������е���������Br2���������������ʺϵ��� ��������ĸ��
����a��NaI b��NaOH c��NaHSO3 d��KCl
��5�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ������������� �������Ʊ�1���嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ���� |
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g?cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
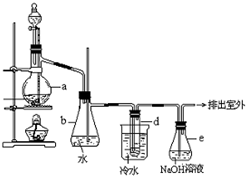 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ����ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4��C���ܶ�Ϊ1.43g?ml-1��
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ����ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4��C���ܶ�Ϊ1.43g?ml-1���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com