
���� ��1��Ba��OH��2��Һ��NaHSO4��Һ��Ӧ����Һ�����ԣ��������ᱵ�������ƺ�ˮ��
��2������������������̼��ơ�̼�����ƺ�ˮ��
��3���������ƹ�����Ӧ���������ƺ�ƫ�����ƣ�
�����йص����ӷ���ʽ��д����ؼ�Ϊ����ȷ��Ӧʵ�ʣ����ٶ��࣬���ٵ�������ȫ��Ӧ��
��� �⣺��1��Ba��OH��2��Һ��NaHSO4��Һ��Ӧ����Һ�����ԣ��������ᱵ�������ƺ�ˮ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
�ʴ�Ϊ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
��2��Ca��HCO3��2��Һ������NaOH��Һ��Ӧ���ӷ��̣�Ca2++HCO3-+OH-�TCaCO3��+H2O��
�ʴ�Ϊ��Ca2++HCO3-+OH-�TCaCO3��+H2O��
��3��Al2��SO4��3��Һ�����NaOH��Һ��Ӧ���ӷ���ʽ��Al3++4OH-�TAlO2-+2H2O��
�ʴ�Ϊ��Al2��SO4��3��Һ�����NaOH��Һ��Ӧ���ӷ���ʽ��
�����йص����ӷ���ʽ��д����ؼ�Ϊ����ȷ��Ӧʵ�ʣ����ٶ��࣬���ٵ�������ȫ��Ӧ��
�ʴ�Ϊ����ȷ��Ӧʵ�ʣ����ٶ��࣬���ٵ�������ȫ��Ӧ��
���� ���⿼�������ӷ���ʽ����д�����ؿ��������йص����ӷ���ʽ��д��Ϊ�߿��ȵ㣬��ȷ��Ӧʵ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ��ȩ | ���� | ����[X& | �Ҷ��� | ˮ |
| �е� | 20.8�� | 117.9�� | 290�� | 197.2�� | 100�� |
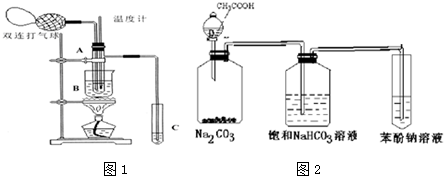
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��1.2NA�����ӵĹ���Na2O2����ˮ���1L��Һ��������Һ��Na+�����ʵ���Ũ��Ϊ0.6mol•L-1 | |
| B�� | Na�ڿ����г��ڷ��ñ��Na2CO3��ĩ | |
| C�� | �����ij���ʯ��ˮ�ֱ���Na2CO3��Һ��NaHCO3��Һ��Ӧ������ͬ | |
| D�� | �μ�KSCN�Ժ�ɫ����Һ�пɴ�������NH4+��K+��Cl-��I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
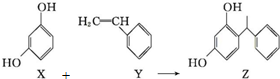
| A�� | X��Y��Z����ʹ��ˮ��ɫ | |
| B�� | Y���ܷ���ȡ����Ӧ��Ҳ�ܷ����ӳɷ�Ӧ | |
| C�� | X��Z������NaHCO3��Һ��Ӧ�ų�CO2 | |
| D�� | Y�����Ӿ۷�Ӧ���壬X�������۷�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
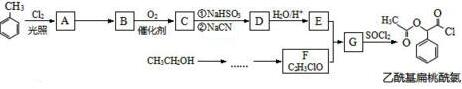
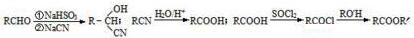
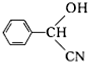 ��
��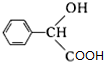 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +nH2O��
+nH2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���³�ѹ�£�16g O2��32g O3�Ļ�����У�����Oԭ������Ϊ3NA | |
| B�� | ��״���£�1mol Na2O��1mol Na2O2�Ļ�����У�������������Ϊ7NA | |
| C�� | 1 mol NaBH4�����Ӽ�����ĿΪ2NA | |
| D�� | ��K${\;}_{\;}^{35}$ClO3+6H${\;}_{\;}^{37}$Cl�TKCl+3Cl2��+3H2O�У�������71 g Cl2��ת�Ƶĵ�����ĿΪ$\frac{5}{3}$NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
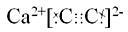
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
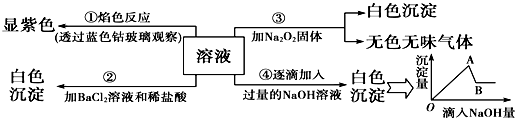
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com