
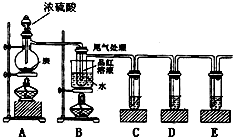
ijĶ¬Ń§ĪŖĮĖ¼ģŃéÅØĮņĖįÓėľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦²śÉśµÄĖłÓŠĘųĢå²śĪļ£¬Ń”ÓĆĮĖĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ”£
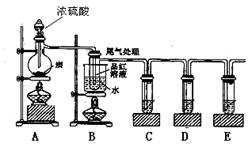
£Ø1£©Š“³öÅØĮņĖįŗĶľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½
____________________________________________________________________ӣ
£Ø2£©ČōÖ¤Ć÷ÓŠĖ®Éś³É£¬ŠčŅŖŌŚA”¢BÖ®¼ä¼Ó×°Ź¢ÓŠ”””””” __£ØĢī»ÆѧŹ½£©¹ĢĢåµÄøÉŌļ¹Ü
£Ø3£©CÖŠĖįŠŌKMnO4ČÜŅŗµÄ×÷ÓĆŹĒ______________________________________”£
£Ø4£©DÖŠÓƵ½Ę·ŗģČÜŅŗ£¬ĖüµÄ×÷ÓĆŹĒ ””””””””””””””””””””””
£Ø5£©EÖŠ³öĻÖµÄĻÖĻóŹĒ”””””””””””””””””””””””””””” £¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ”””””””””””””””””””””””””””””””””””””””””””””” ”£
(6)×°ÖĆBæÉŅŌĢ½¾æSO2ÓėĘ·ŗģ×÷ÓƵÄæÉÄęŠŌ£¬ĒėŠ“³öŹµŃé²Ł×÷¼°ĻÖĻó_______””””””
___________________________________________________________________________ӣ
 æŚĖćÄÜŹÖĻµĮŠ“š°ø
æŚĖćÄÜŹÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 CO2ӟ+2SO2ӟ+2H2O
CO2ӟ+2SO2ӟ+2H2O CO2ӟ+2SO2ӟ+2H2O
CO2”ü+2SO2”ü+2H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”Ģ©°²ÄžŃō¶žÖŠø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
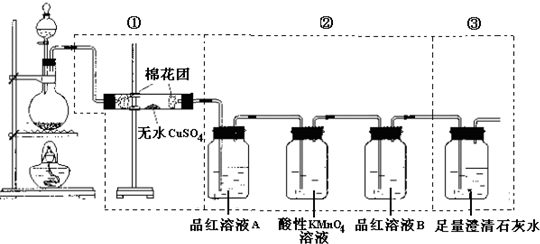
£Ø12·Ö£©Ä³Ķ¬Ń§ĪŖĮĖ¼ģŃéÅØĮņĖįÓėľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦²śÉśµÄĖłÓŠĘųĢå²śĪļ£¬Ń”ÓĆĮĖĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ”£
£Ø1£©Š“³öÅØĮņĖįŗĶľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½
___________________________________________________ӣ
£Ø2£©¢ŁÖŠĪŽĖ®ĮņĖįĶµÄ×÷ÓĆŹĒ ”£
£Ø3£©¢ŚÖŠĖįŠŌKMnO4ČÜŅŗµÄ×÷ÓĆŹĒ______________________”£
£Ø4£©¢ŚÖŠĮ½“ĪÓƵ½Ę·ŗģČÜŅŗ£¬ĖüĆĒµÄ×÷ÓĆ·Ö±šŹĒA ”¢B ”£
£Ø5£©¢ŪÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Ä³Ķ¬Ń§ĪŖĮĖ¼ģŃéÅØĮņĖįÓėľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦²śÉśµÄĖłÓŠĘųĢå²śĪļ£¬Ń”ÓĆĮĖĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ”£
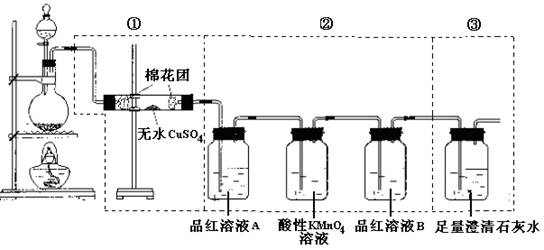
£Ø1£©Š“³öÅØĮņĖįŗĶľĢæ·ŪŌŚ¼ÓČČĢõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½
___________________________________________________ӣ
£Ø2£©¢ŁÖŠĪŽĖ®ĮņĖįĶµÄ×÷ÓĆŹĒ ”£
£Ø3£©¢ŚÖŠĖįŠŌKMnO4ČÜŅŗµÄ×÷ÓĆŹĒ______________________”£
£Ø4£©¢ŚÖŠĮ½“ĪÓƵ½Ę·ŗģČÜŅŗ£¬ĖüĆĒµÄ×÷ÓĆ·Ö±šŹĒA ”¢B ”£
£Ø5£©¢ŪÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com