
(ĆææÕ2·Ö£¬¹²10·Ö)A”¢B”¢C”¢D”¢E¶¼ŹĒ¶ĢÖÜĘŚŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĪåÖÖŌŖĖŲŗĖµēŗÉ×ÜŹżĪŖ42£¬B”¢CĶ¬ÖÜĘŚ£¬A”¢DĶ¬Ö÷×唣A”¢BÄÜŠĪ³ÉĮ½ÖÖŅŗĢ¬»ÆŗĻĪļ¼×ŗĶŅŅ£¬Ō×ÓøöŹż±Č·Ö±šĪŖ2”Ć1ŗĶ1”Ć1”£øł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å¼×”¢ŅŅĮ½·Ö×ÓÖŠŗ¬ÓŠ·Ē¼«ŠŌ¹²¼Ū¼üµÄĪļÖŹµÄµē×ÓŹ½ŹĒ £¬CŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ ”£
¢ĘCŗĶDµÄĄė×ÓÖŠ£¬°ė¾¶½ĻŠ”µÄŹĒ £ØĢīĄė×Ó·ūŗÅ£©”£
¢Ē½«DµÄµ„ÖŹĶ¶Čė¼×ÖŠ£¬“żDĻūŹ§ŗóŌŁĻņÉĻŹöČÜŅŗÖŠ¼ÓČėEµÄµ„ÖŹ£¬“ĖŹ±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ______________________________________________________”£
¢ČC”¢D”¢EæÉ×é³ÉĄė×Ó»ÆŗĻĪļDxEC6£¬Ę侧°ū£Ø¾§°ūŹĒŌŚ¾§ĢåÖŠ¾ßÓŠ“ś±ķŠŌµÄ×īŠ”ÖŲø“µ„ŌŖ£©½į¹¹ČēĻĀĶ¼ĖłŹ¾£¬ŃōĄė×ÓD£«£ØÓĆ”š±ķŹ¾£©Ī»ÓŚÕż·½ĢåµÄĄāµÄÖŠµćŗĶÕż·½ĢåÄŚ²æ£»ŅõĄė×ÓEC6x££ØÓĆ”ń±ķŹ¾£©Ī»ÓŚøĆÕż·½ĢåµÄ¶„µćŗĶĆęŠÄ”£øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ŹĒ ”£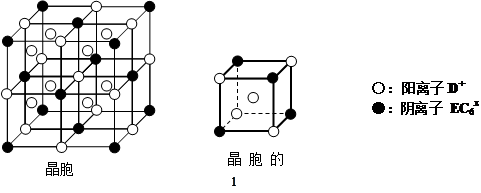
 ĒįĖÉæĪĢƵ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø
ĒįĖÉæĪĢƵ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£ØĆææÕ2·Ö£¬¹²10·Ö£©ĪļÖŹFŹĒŗĻ³É¶ąÖÖŹÖŠŌŅ©ĪļŗĶÉśĪļ»īŠŌĪļÖŹµÄÖŲŅŖÖŠ¼äĢ壬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
£Ø1£©·“Ó¦¢ŁµÄĄąŠĶĪŖ £¬·“Ó¦¢ÜµÄĄąŠĶĪŖ ”£
£Ø2£©ĪļÖŹA¾ŪŗĻæÉµĆ¾Ū±ūČ²Ėį¼×õ„£¬Ęä½į¹¹¼ņŹ½ĪŖ ”£
£Ø3£©ĪļÖŹCµÄĖ×ĆūĪŖČā¹šČ©£¬Š“³öĘäĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£
¢ŁÄÜÓėĀČ»ÆĢśČÜŅŗĻŌ×ĻÉ«£»¢Ś±½»·ÉĻÓŠĮ½øöČ”“ś»ł£»¢Ū·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·å”£
£Ø4£©Čō²æ·ÖC±»Ńõ»ÆĪŖČā¹šĖį£¬·“Ó¦¢Ś½«²śÉśø±²śĪļ£Ø·Ö×ÓŹ½ĪŖC14H15NO3£©£¬Š“³öĘä½į¹¹¼ņŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÖŲĒģŹŠŃī¼ŅĘŗ֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²10·Ö£©ŅĄ¾ŻŹĀŹµ£¬ĢīæÕ£ŗ
£Ø1£©ŅŃÖŖijĢõ¼žĻĀæÉ·¢ÉśČēĻĀ·“Ó¦CH3£CH3”śCH2£½CH2£«H2£¬ÓŠ¹Ų»Æѧ¼üµÄ¼üÄÜČēĻĀ£ŗ
| »Æѧ¼ü | C£H | C£½C | C£C | H£H |
| ¼üÄÜ£ØkJ/mol£© | 414.4 | 615.3 | 347.4 | 435.3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŌĘÄĻŹ”ÓńĻŖŅ»ÖŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²10·Ö£©Ź³Ę·°²Č«¹ŲĻµ¹ś¼ĘĆńÉś£¬Ó°ĻģŹ³Ę·°²Č«µÄŅņĖŲŗܶą”£
(1)¾ŪĘ«¶žĀČŅŅĻ©(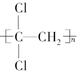 )¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
)¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
(2)ĮÓÖŹÖ²ĪļÓĶÖŠµÄŃĒÓĶĖį[CH3(CH2)4”ŖCH===CH”ŖCH2”ŖCH===CH”Ŗ(CH2)7COOH]ŗ¬ĮæŗܵĶ”£ĻĀĮŠ¹ŲÓŚŃĒÓĶĖįµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________”£
| A£®·Ö×ÓŹ½ĪŖC18H34O2 |
| B£®Ņ»¶ØĢõ¼žĻĀÄÜÓėŅŅĖį·¢Éśõ„»Æ·“Ó¦ |
| C£®ÄÜŗĶNaOHČÜŅŗ·“Ó¦ |
| D£®ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŌĘÄĻŹ”ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²10·Ö£©Ź³Ę·°²Č«¹ŲĻµ¹ś¼ĘĆńÉś£¬Ó°ĻģŹ³Ę·°²Č«µÄŅņĖŲŗܶą”£
(1)¾ŪĘ«¶žĀČŅŅĻ©( )¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
)¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
(2)ĮÓÖŹÖ²ĪļÓĶÖŠµÄŃĒÓĶĖį[CH3(CH2)4”ŖCH===CH”ŖCH2”ŖCH===CH”Ŗ(CH2)7COOH]ŗ¬ĮæŗܵĶ”£ĻĀĮŠ¹ŲÓŚŃĒÓĶĖįµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________”£
A£®·Ö×ÓŹ½ĪŖC18H34O2
B£®Ņ»¶ØĢõ¼žĻĀÄÜÓėŅŅĖį·¢Éśõ„»Æ·“Ó¦
C£®ÄÜŗĶNaOHČÜŅŗ·“Ó¦
D£®ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«
(3)¼Ł¾ĘÖŠ¼×“¼(CH3OH)ŗ¬Į泬±ź£¬ĒėŠ“³öNaŗĶ¼×“¼·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ____________________”£
(4)ĮÓÖŹÄĢ·ŪÖŠµ°°×ÖŹŗ¬ĮæŗܵĶ”£µ°°×ÖŹĖ®½āµÄ×īÖÕ²śĪļŹĒ__________________”£
(5)ŌŚµķ·ŪÖŠ¼ÓČėµõ°×æéÖʵƵķŪĖæÓŠ¶¾”£µķ·Ū×īÖÕµÄĖ®½ā²śĪļŹĒĘĻĢŃĢĒ”£ĒėÉč¼ĘŹµŃéÖ¤Ć÷µķ·ŪŅŃ¾Č«²æĖ®½ā£¬Š“³ö²Ł×÷”¢ĻÖĻóŗĶ½įĀŪ£ŗ_________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«¹ŚĻŲĪäѵøßÖŠø߶žĻĀѧʌµŚČż“Īæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²10·Ö£©
ijĢžA 0.2 mol ŌŚŃõĘųÖŠĶźČ«Č¼ÉÕŗó£¬Éś³ÉCO2ŗĶH2Oø÷1.2 mol”£ŹŌ»Ų“š£ŗ
£Ø1£©ĢžAµÄ·Ö×ÓŹ½ĪŖ_____________”£
£Ø2£©ČōČ”Ņ»¶ØĮæµÄĢžAĶźČ«Č¼ÉÕŗó£¬Éś³ÉCO2ŗĶH2Oø÷3 mol£¬ŌņÓŠ________gĢžA²Ī¼ÓĮĖ·“Ó¦£¬Č¼ÉÕŹ±Ļūŗıź×¼×“æöĻĀµÄŃõĘų___________L”£
£Ø3£©ČōĢžA²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČČ”“śĪļÖ»ÓŠŅ»ÖÖ£¬ŌņĢžAµÄ½į¹¹¼ņŹ½ĪŖ__________________”£
£Ø4£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬ÓėH2¼Ó³É£¬Ęä¼Ó³É²śĪļ¾²ā¶Ø·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬ĢžAæÉÄÜÓŠµÄ½į¹¹¼ņŹ½ĪŖ______________£ØŠ“³öŅ»ÖÖ¼“æÉ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com