
���� | ��ѧʽ | ��ɫ��״̬ | �ܽ��(g) | �۵�(��) | �ܶ�(g��cm3) |
�Ҷ��� | H | �� | 8.6(20��) | 189.5 | 1.900 |
��ˮ���Ҷ��� | H | ��ɫ���� | �� | 101.5 | 1.650 |
ע���Ҷ���(HOOC��COOH)�׳Ʋ��ᣬ��2���ᾧˮ���Ҷ����׳Ʋ��ᾧ�壬���ᾧ��ʧȥ�ᾧˮ����ˮ���ᣬ��Լ157��ʱ���������β���ƺͲ�����ƾ�Ϊ��ɫ���������������Ϣ���ش��������⡣
(1)��ʢ��Na2CO3��ĩ���Թ������Լ3 mL�Ҷ�����Һ���۲쵽��������________________��
(2)��ʢ��5 mL�Ҷ��ᱥ����Һ���Թ������3���������ữ��0.5��(��������)�ĸ��������Һ�����۲쵽������Ϊ____________��˵���Ҷ�����_____________�ԡ�
(3)��֪����ֽ�Ļ�ѧ����ʽΪ��H![]() H2O+CO2��+CO��������ͼװ�ü��Ȳ��ᾧ�壬��֤�������ȷֽ⼰������������Ϊ��װ�ò�������������ݲ��ᾧ���ijЩ����������ʵ��Ŀ�ģ�����������ԭ��_____________(�����������ѡ��)��
H2O+CO2��+CO��������ͼװ�ü��Ȳ��ᾧ�壬��֤�������ȷֽ⼰������������Ϊ��װ�ò�������������ݲ��ᾧ���ijЩ����������ʵ��Ŀ�ģ�����������ԭ��_____________(�����������ѡ��)��
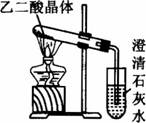
a.���ᾧ����۵�ͣ�δ�ֽ�֮ǰ�����ڻ��������Թܿڣ������ڻ�ѧ��Ӧ�Ľ��м�ʵ������Ĺ۲�
b.��Ϊ���ȵ��Dz��ᾧ�壬���Ը�װ�����ֻ�ܼ���һ�ַֽ������ܼ����Ƿ��������ֽ����
C.��δϴ���������ͨ��ʯ��ˮ�������������������ʯ��ˮ��Ӧ���ɲ���Ƴ�������ʵ���и���
(4)��װ��Ҳ��������ɫ��ѧ��Ҫ����˵��ԭ��____________________________��
(5)ʵ���������ζ����ⶨ���ᾧ������нᾧˮ�����ķ������£���ȡa g���ᾧ������ˮ���200 mL��Һ��ȡ20 mL����Һ��0.1 mol��L-1NaOH��Һ�ζ������ﵽ�յ�ʱ����ȥNaOH��Һb mL����ÿ�����ᾧ������нᾧˮ����x=__________(����ĸ��ʾ��ʽ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ɷ� | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | CaS | ����������������� |
| ����������%�� | 65��66 | 3.5��5.0 | 1.5��3.5 | 0.2��0.8 | 0.2��1.1 | 1.0��1.8 | 23��26 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪һЩ���ε���ɫ���ܽ����ֵ���£�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���Ჴ������Ľṹ��ʽΪ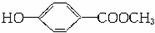 ��д���Ჴ�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ���л���д�ṹ��ʽ����_____________________________________________________��
��д���Ჴ�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ���л���д�ṹ��ʽ����_____________________________________________________��
��2���Ჴ�������һ��ͬ���칹��A�����������������ٺ��б������ں���̼̼˫�����۱�����һ��ȡ����ֻ��һ�֡�д��A�Ŀ��ܵĽṹ��ʽ��_____________________________��
��3���Ჴ���������һ��ͬ���칹��B���䱽���ϵ�ȡ�����˴���䣬������ת����ϵ��
![]()
��֪C�Ļ�ѧʽΪC8H7O3Na��F�Ļ�ѧʽΪC8H6O3����д���пհף�
��B�еĹ�����������_____________��E��F�ķ�Ӧ����Ϊ_____________����F�Ľṹ��ʽΪ______________����д��E���Ҵ���Ӧ�Ļ�ѧ����ʽ���л���д�ṹ��ʽ����ע����Ӧ��������____________________________________________________________________��
��4����W g�Ჴ�������������������ϵ�ȼ����ַ�Ӧ������a g������̼��b gˮ�����Ჴ���������Է�������ΪM����ȷ���Ჴ�������������ԭ�����ļ���ʽ�����ػ���Ϊ____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ��ѧʽ | ��ɫ��״̬ | �ܽ�ȣ�g�� | �۵㣨�棩 | �ܶȣ�g/cm3�� |
| �Ҷ��� | H2C2O4 | �D | 8.6��20�棩 | 189.5 | 1.900 |
| ��ˮ���Ҷ��� | H2C22?2H2O | ��ɫ���� | �D | 101.5 | 1.650 |
ע���Ҷ��ᣨHOOC�DCOOH���׳Ʋ��ᣬ��2���ᾧˮ���Ҷ����׳Ʋ��ᾧ�壬���ᾧ��ʧȥ�ᾧˮ����ˮ���ᣬ��Լ157��ʱ���������β���ƺͲ�����ƾ�Ϊ��ɫ�����
����������Ϣ���ش��������⡣
��1����ʢ��Na2CO3��ĩ���Թ������Լ3mL�Ҷ�����Һ���۲쵽�������� ��˵���Ҷ�������Ա�̼�� ���ǿ������������
��2����ʢ��5mL�Ҷ��ᱥ����Һ���Թ��е���3���������ữ��0.5%�������������ĸ��������Һ�����۲쵽������Ϊ _______ ��˵���Ҷ�����_____ �ԡ�
��3����A�Թ��м���3mL�Ҷ��ᣬȻ������Թܱ���2mLŨ�����2mL�Ҷ��ᣬ����ͼ�����Ӻ�װ�ã�����3 -5min����B��������״�Ҵ�����ζ��Һ�������������˵�������� ��Ӧ�����л���Ӧ���ͣ�����B�Թ��м��뱥�� Na2CO3��Һ��Ŀ���� ��
B�е��ܿ���Һ���ϵ�ԭ���� ��
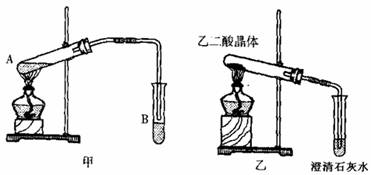
��4����֪����ֽ�Ļ�ѧ����ʽΪ��H2C2O4 ![]() H2O+CO2��+CO������ͼ��װ�ü��Ȳ��ᾧ�壬��֤�������ȷֽ⼰������������Ϊ��װ�ò�������������������Ϣ��ʵ��Ŀ�ģ�����������ԭ�� �������������ѡ��
H2O+CO2��+CO������ͼ��װ�ü��Ȳ��ᾧ�壬��֤�������ȷֽ⼰������������Ϊ��װ�ò�������������������Ϣ��ʵ��Ŀ�ģ�����������ԭ�� �������������ѡ��
a�����ᾧ����۵�ϵͣ�δ�ֽ�֮ǰ�����ڻ��������Թܿڣ������ڻ�ѧ��Ӧ�Ľ��м�ʵ������Ĺ۲졣
b����Ϊ�����Dz��ᾧ�壬���Ը�װ�����ֻ�ܼ���һ�ַֽ������ܼ����Ƿ�
���������ֽ���
c����δϴ���������ͨ��ʯ��ˮ�������������������ʯ��ˮ��Ӧ���ɲ���Ƴ�������ʵ���и��š�
��5����װ��Ҳ��������ɫ��ѧ��Ҫ����˵��ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com