
��22�֣�2SO2(g)+O2(g) 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=��99kJ��mol��1����ش��������⣺
��1��ͼ��A��C�ֱ��ʾ �� ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��2��ͼ�С�H= KJ��mol��1��
��3��V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ ��
��4����2�֣������Ӧ���ʦԣ�SO2��Ϊ0��05 mol��L��1��min��1,��ԣ�O2��= mol��L��1��min��1��
��(SO3)= mol��L��1��min��1��
��5����2�֣���֪�������ȼ����Ϊ296 KJ��mol��1��������S(s)����3 molSO3(g)�ġ�H ��Ҫ�������̣���
��6������ȼ�ϵ�� ��KOH���������Һ��
������Ӧ����ʽ�ǣ�
������Ӧ����ʽ�ǣ�
�ܷ�Ӧ����ʽ�ǣ�
��7����2�֣������£���pH ��Ϊ5��H2SO4��A12(SO4)3��Һ����ˮ�������c(H+)�ֱ�Ϊc1 ��c2����c1��c2= ��
��8����2�֣�Ũ��Ϊ0.5 mol/L���������Ũ�ȵİ�ˮ��Һ��Ӧ��ʹ��Һ�����ԣ�����ǰ���V��________V��(����ڡ�����С�ڡ����ڡ�)
(9)��2�֣�ȡ10 mL��Һ0.5 mol/L�����ᣬ��ˮϡ�͵�500 mL�����ʱ��Һ����ˮ�������c(H��)��________��
��22�֣���1����Ӧ������ ���������� �� ���� ��Ϊ�����ı��˷�Ӧ������ʹ���E���� ��2����2�֣���198
(3) ��2�֣�SO2+V2O5=SO3+2VO2 4VO2+O2=2V2O5
(4)��2�֣�0��025 0��05
(5) ��2�֣�S(s)+O2(g)=2SO2(g) ��H1=��296KJ��mol��1 ,
SO2(g)+1/2O2(g) SO3(g)��H2=��99 KJ��mol��1
3 S(s)+9/2O2(g)=3SO3(g) ��H=3(��H1+��H2)=��1185 KJ��mol��1
��6��������CH4+10OH- ��8e-=CO32-+7H2O ������O2��2H2O+4e-=4OH-
�ܷ�Ӧ��CH4��2 O2+2OH-=CO32-+3H2O
��7����2�֣�1��104 ��8����2�֣�С�ڣ�9����2�֣�10-12
����:


 �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.Q=Q1/2 B.Q2��Q1/2 C.Q2��Q1��Q D.Q=Q1��Q2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£���һ���������ܱ������г���2 mol SO2��1 mol O2���������з�Ӧ��2SO2 (g)+O2 (g) 2SO3(g)���ﵽƽ���ı�����������SO3����ƽ��Ũ�Ȳ��ı���ǣ� ��
A.�����¶Ⱥ�����������䣬����1 mol SO3 (g)
B.�����¶Ⱥ�������ѹǿ���䣬����1 mol SO3 (g)
C.�����¶Ⱥ�������ѹǿ���䣬����1 mol O2(g)
D.�����¶Ⱥ�������ѹǿ���䣬����1 mol Ar(g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��) �Ӵ��������Ṥ���У�������Ӧ��450�沢�д��������½��У�
2SO2(g)+O2(g) 2SO3(g) ��H=��190 kJ��mo1��1
��1����һ�̶������г���2mol SO2��1molO2 ����һ���������´ﵽƽ�⣬��Ӧ�ų�������__________(����ڡ�С�ڻ����) 190 kJ
��2����һ���̶��ݻ�Ϊ5L���ܱ������г���0.20mol SO2��0.10molO2������Ӻ�ﵽƽ�⣬��������к�SO30.18mol����v(O2)=______mol��L-1��min-1
��3�����������ĸı���䷴Ӧ���ʼ�����Ӱ����� (ѡ�����)
�������¶� �ڱ���������䣬ֻ�������������� �۱���������䣬����Neʹ��ϵѹǿ���� �ܱ���ѹǿ���䣬����Neʹ�������������
��4��������������˵������(1)��Ӧ�Ѵ�ƽ����� (ѡ�����)
����v(O2)����2v(SO3)������SO2��O2 ��SO3��Ũ��֮��Ϊ2��1��2
�۵�λʱ��������2n molSO2��ͬʱ����2n mol SO3
�������������ƽ������������ʱ����仯
������������������ܶȲ���ʱ����仯 ������������ѹǿ����ʱ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�������������и߶�3���¿���ѧ�Ծ� ���ͣ������
��22�֣�2SO2(g)+O2(g)  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol�Ħ�H=��99kJ��mol��1����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol�Ħ�H=��99kJ��mol��1����ش��������⣺
��1��ͼ��A��C�ֱ��ʾ �� ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��2��ͼ�С�H= KJ��mol��1��
��3��V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ ��
��4����2�֣������Ӧ���ʦԣ�SO2��Ϊ0��05 mol��L��1��min��1,��ԣ�O2��= mol��L��1��min��1��
��(SO3)= mol��L��1��min��1��
��5����2�֣���֪�������ȼ����Ϊ296 KJ��mol��1��������S(s)����3 molSO3(g)�ġ�H ��Ҫ�������̣���
��6������ȼ�ϵ�أ�KOH���������Һ��
������Ӧ����ʽ�ǣ�
������Ӧ����ʽ�ǣ�
�ܷ�Ӧ����ʽ�ǣ�
��7����2�֣������£���pH ��Ϊ5��H2SO4��A12(SO4)3��Һ����ˮ�������c(H+)�ֱ�Ϊc1��c2����c1��c2= ��
��8����2�֣�Ũ��Ϊ0.5 mol/L���������Ũ�ȵİ�ˮ��Һ��Ӧ��ʹ��Һ�����ԣ�����ǰ���V��________V��(����ڡ�����С�ڡ����ڡ�)
(9)��2�֣�ȡ10 mL��Һ0.5 mol/L�����ᣬ��ˮϡ�͵�500 mL�����ʱ��Һ����ˮ�������c(H��)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ�����УЭ����߶���ѧ������������ѧ�Ծ����������� ���ͣ������
(12��)�ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɶ����ѵ����ȼ�ϡ�����Ȼ����úϳ��������п��ܷ����ķ�Ӧ�У�
��CH4(g)+H2O(g) CO(g)+3H2(g) �SH1="+206.1" kJ/mol
CO(g)+3H2(g) �SH1="+206.1" kJ/mol
��CH4(g)+CO2(g) 2CO(g)+2H2(g) �SH2="+247.3" kJ/mol
2CO(g)+2H2(g) �SH2="+247.3" kJ/mol
��CO(g)+H2O(g) CO2(g)+ H2(g) �SH3
CO2(g)+ H2(g) �SH3
��ش��������⣺
(1)��һ�ܱ������н��з�Ӧ�٣���� �����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ��10minʱ���ı��������������� ��
�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ��10minʱ���ı��������������� ��
(2)��ͼ2��ʾ���ڼס����������зֱ��������ʵ����� ��
�� ��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������
��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪�������� ��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л�����������
��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л����������� ��ת������ʱ��仯��ͼ��
��ת������ʱ��仯��ͼ��
(3)��Ӧ����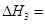 ��800��ʱ����Ӧ�۵Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
��800��ʱ����Ӧ�۵Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
��ʱ��Ӧ���������淴Ӧ���ʵĹ�ϵʽ�� (�����)��
a�� (��)
(��) (��) b��
(��) b�� (��)<
(��)< (��) c��
(��) c�� (��)=
(��)= (��) d�����ж�
(��) d�����ж�
��4��800Kʱ������ʼ�����ͬ���ܱ������г���2mol SO2��1mol O2���䷴Ӧ��2SO2(g)+O2(g)  2SO3(g)����H=��96.56 kJ?mol-1���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������ά�־��ȣ����������Խ�����ѧƽ�⡣
2SO3(g)����H=��96.56 kJ?mol-1���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������ά�־��ȣ����������Խ�����ѧƽ�⡣
��1���ﵽƽ��ʱ��ƽ�ⳣ��K (��) K (��) K(��)���>������<����=������
��2���ﵽƽ��ʱSO2��Ũ��C(SO2)(��) C(SO2) (��) C(SO2) (��)���>������<����=������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com