
A��B��C��DΪ���ֶ�����Ԫ�أ�ԭ��������������ԭ�Ӱ뾶��C��D��B��A��˳����С��A��Cλ��ͬһ���壬B��Dλ��ͬһ���壻A��C��ԭ�Ӻ���������֮��ΪB��D��ԭ�Ӻ���������֮�͵�һ�룻CԪ������������Ԫ�ؾ����γ����ӻ����
�Իش��������⣺
(1)B�����ӷ���Ϊ________��C��D�γɵĻ�����ĵ���ʽΪ________��
(2)ͬʱ������������Ԫ�صĻ������ж��֣�д����������һ�ֻ�����Ļ�ѧʽ__________________________________________��
(3)��A��B��C�е�����Ԫ����ɵ����ֻ��������Ӧ����A���ʵĻ�ѧ����ʽΪ__________________________________��
(4)��B��C��D�е�����Ԫ����ɵ����ֻ�������ķ�Ӧ�����ڻ��Ϸ�Ӧ������������ԭ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ______��
�𰸡�(1)O2����Na��[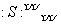 ]2��Na����(2)Na2SO4·10H2O��NaHSO4��NaHSO3(��дһ�ּ���)
]2��Na����(2)Na2SO4·10H2O��NaHSO4��NaHSO3(��дһ�ּ���)
(3)NaH��H2O===NaOH��H2��
(4)Na2O2��SO2===Na2SO4
������CԪ������������Ԫ�ؾ����γ����ӻ������CΪ���ý�������A��Cλ��ͬһ���壬AӦΪ�ǽ�������AΪH��CΪNa����B��ԭ������Ϊx����D��ԭ������ӦΪx��8��A��C��ԭ�Ӻ���������֮��ΪB��D��ԭ�Ӻ���������֮�͵�һ�룬����x��x��8��2��(1��11)��x��8������BΪO��DΪS��
(1)BΪO����Ӧ������ΪO2����DΪS��C��D�γɵĻ�����Na2S��Ϊ���ӻ��������ʽΪ
Na��2��Na����
(2)��H��O��Na��S��ɵĻ�������Na2SO4·10H2O��NaHSO4��NaHSO3�ȡ�
(3)��A��B��C�е�����Ԫ����ɵĻ�������NaH��H2O��Na2O��Na2O2�ȣ������ܷ�Ӧ����H2��ΪNaH��H2O����Ӧ�Ļ�ѧ����ʽΪNaH��H2O===NaOH��H2����
(4)��B��C��D�е�����Ԫ����ɵĻ�������Na2O2��Na2O��SO2��SO3�ȣ������ķ�Ӧ�����ڻ��Ϸ�Ӧ������������ԭ��Ӧ��ΪNa2O2��SO2����Ӧ�Ļ�ѧ����ʽΪNa2O2��SO2===Na2SO4��


 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ
�� A��Ũ��������м��Ӧ��2Fe + 6H+ = 2Fe3+ + 3H2��
�� B��Cl2ͨ��ˮ�У�Cl2 + H2O = 2H��+ Cl��+ ClO��
�� C����ϡ�����е�������������Һ��Ba2++SO42-=BaSO4��
�� D��ʵ�����Ʊ�������̼���壺 2H+ + CaCO3 �T Ca2+ + CO2��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����е����NH3��CO2��NO�Ļ����������ͨ��ʢ��Ũ���ᡢNa2O2��Na2CO3��Һ��װ�ã�������ú����õ���������(����)
A��CO2��NO B��CO2
C��NO D��NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijԪ������������Ӧ��ˮ����Ļ�ѧʽΪH2XO4����Ԫ�ص���̬�⻯��Ļ�ѧʽ��(����)
A��HX B��H2X
C��XH3 D��XH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鲻����Ϊ�ж����ݵ���(����)
A���ƺ�þ�ֱ�����ˮ��Ӧ���ж��ƺ�þ������ǿ��
B����MgCl2��AlCl3��Һ�зֱ���������NaOH��Һ���ж�þ�����Ľ�����ǿ��
C������������Һ��ͨ��CO2������ɫ�������ж�̼������������ǿ��
D������HF��HCl��ˮ��Һ������ǿ���жϷ����ȵķǽ����Ե�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������˵����ȷ����(����)
A��1 L 1 mol·L��1��NaClO��Һ�к���ClO������ĿΪNA
B��78 g������C===C������ĿΪ3 NA
C�����³�ѹ�£�14 g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA
D����״���£�6.72 L NO2��ˮ��ַ�Ӧת�Ƶĵ�����ĿΪ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ0.1 mol·L��1 NaOH��Һ450 mL��0.5 mol·L��1������Һ500 mL��������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________________(����������)��
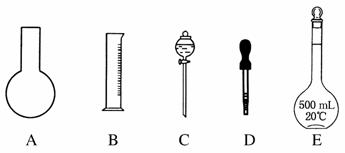
(2)���в����У�����ƿ�����߱��Ĺ�����________(�����)��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E����ȡһ�������Һ��
F�����������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ______g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________(����ڡ��������ڡ���С�ڡ�����ͬ)0.1 mol·L��1����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________0.1 mol·L��1��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g·cm��3��Ũ��������Ϊ________ mL(����������һλС��)�����ʵ������15 mL��20 mL��50 mL��Ͳ��Ӧѡ��________mL����Ͳ��á����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ����������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ̽����ѧϰС������ͼ��ʾ��װ�ý���ʵ�飬�����ºͱ�����ѹǿ�£����a g��Al 96%����Ʒ��(��Ʒ���е����ʲ���ϡ���ᷴӦ)��ϡ������ȫ��Ӧ�������������Ϊb L����������ͬ������ ����NaOH��Һ�����װ���е�ϡ�������ⶨijһ��Ʒ����Al����������(��Ʒ���е����ʲ���NaOH��Һ��Ӧ)����ش��������⣺
����NaOH��Һ�����װ���е�ϡ�������ⶨijһ��Ʒ����Al����������(��Ʒ���е����ʲ���NaOH��Һ��Ӧ)����ش��������⣺
��1��Al��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��
�� .
��2������װ��ͼ�����Ӻ�װ�ã����װ�õ������ԣ�����ҩƷ��ʹ��Ӧ��ʼ�����IJ����ǡ�
��  .
.
��3����ʵ���в���������� ʱӦע��������С�
ʱӦע��������С�
�� .
��4�������������ɵ������Ħ�������������(����ڡ�����С�ڡ����ڡ�)22.4 L/mol.
��5 ���������Ʒ�ҵ�����Ϊc g�������������Ϊd L��������Ʒ����Al��������������ʽ w(Al)����������.
���������Ʒ�ҵ�����Ϊc g�������������Ϊd L��������Ʒ����Al��������������ʽ w(Al)����������.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������ȫ����������ʵ���
A��H2SiO3 H2S CO2 B��MgSO4 CH3COOH CH3CH2OH
C��H2SO3 BaSO4 CH4 D��H2O NH3•H2O H3PO4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com