
��һ�����Ϊ2L���ܱ������У������·������з�Ӧ��
����Fe(s) �� CO2(g) FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ
����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��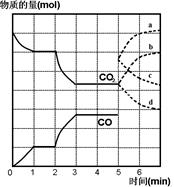
(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
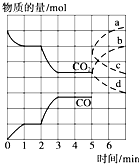 ��һ�����Ϊ2L���ܱ������У������·�����Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g��������CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ϵ��ͼ��ʾ��
��һ�����Ϊ2L���ܱ������У������·�����Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g��������CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�����Ϊ2L���ܱ������У������·������з�Ӧ��
����Fe(s) �� CO2(g)FeO(s) �� CO(g) + Q kJ
����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��
(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ���������������ѧ����ĩ������⻯ѧ�Ծ� ���ͣ������
��һ�����Ϊ2L���ܱ������У������·������з�Ӧ��
����Fe(s) �� CO2(g) FeO(s) �� CO(g) + Q kJ
FeO(s) �� CO(g) + Q kJ
����CO2��CO�����ʵ�����mol����ʱ�䣨min���ı仯��ͼ��ʾ��
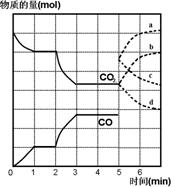
(1)����Ӧ��1minʱ��һ�δﵽƽ��״̬�����������������3.2g����CO��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʦ�(CO)=_________��
(2)����Ӧ������2minʱ����ֻ�ı�һ�����������߷����ı仯��ͼ��ʾ��3minʱ�ٴδﵽƽ�⣬��Q 0�����������������=��������һ��ƽ����ڶ���ƽ���ƽ�ⳣ����ȣ�K1 K2��(���������������=��)��
(3)��5minʱ�ٳ���һ������CO(g)��ƽ�ⷢ���ƶ�������˵����ȷ���� ����д��ţ���
a����(��)��������С b����(��)�ȼ�С������
c����(��)��������С d����(��)�ȼ�С������
��ʾn(CO2)�仯��������________����дͼ�����ߵ���ĸ��ţ���
(4)�����ù�̬���ʵ��й���������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬��
______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A����ͼ��ʾ��ת����ϵ����֪��X��һ�����嵥�ʣ�Y��һ����ɫҺ�壬һ��������D��H��Ũ��Һ���������ͭ��Ӧ��
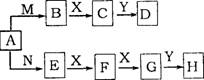
(1)��A��������ˮ������ӷ���ʽ�������������� ��
(2)д��E��F�Ļ�ѧ����ʽ�������������������� ��
(3)ʵ������ͨ����H��Ũ��Һ�������������� �Լ�ƿ�У��ҷ���������
(4)����D��HŨ��Һ�Ļ���ﹲ��ʱ������Ӧ�Ļ�ѧ����ʽ���������������������� ��
(5)��һ�����Ϊ2L�ĺ����ܱ������У�����5mol����B��3mol����X����һ���¶��·�����Ӧ��������C����5min�ﵽƽ�⣬��ʱB��ת����Ϊ80����
�� ����ǰ5min�ڣ�������C��ʾ�Ļ�ѧ��Ӧ����Ϊ�������������� ��
�� ���ڸ��¶��£���Ӧ�Ļ�ѧƽ�ⳣ��K=�������������� ��
�� �۴ﵽƽ������������а����ʵ���1��1��4�ı����ٴγ���B��X��C��B��ת���ʽ������� (���������С���������䡱)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com