��¯������ұ��������Ҫ��������������Ҫ��ӦΪ��
Fe
2O
3��s��+3CO��g��?2Fe��s��+3CO
2��g����H=a kJ?mol
-1��1����֪����Fe
2O
3��s��+3C��ʯī��=2Fe��s��+3CO��g����H
1=+489.0kJ?mol
-1��C��ʯī��+CO
2��g��=2CO��g����H
2=+172.5kJ?mol
-1��a=
kJ?mol
-1��
��2��ұ������Ӧ��ƽ�ⳣ������ʽK=
���¶����ߺ�Kֵ
������������䡱��С������
��3����T��ʱ���÷�Ӧ��ƽ�ⳣ��K=64���� 2L�����ܱ����������У��ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⣮
|
Fe2O3 |
CO |
Fe |
CO2 |
| ��/mol |
1.0 |
1.0 |
1.0 |
1.0 |
| ��/mol |
1.0 |
2.0 |
1.0 |
1.0 |
�ټ�������CO��ƽ��ת����Ϊ
��
������˵����ȷ����
������ĸ����
a���������������ܶȺ㶨ʱ����־��Ӧ�ﵽƽ��״̬
b������Fe
2O
3�������CO��ת����
c����������CO��ƽ��ת���ʴ����ҵ�ƽ��ת����
d���ס��������У�CO��ƽ��Ũ��֮��Ϊ2��3
��4����ȡһ����ʩ�ɷ�ֹ������ʴ������װ���е��ձ����ʢ�е�Ũ�ȡ��������NaCl��Һ��
����abcװ�����ܱ���������
������ĸ��
������dװ�ñ�������X���ĵ缫����Ӧ��
�������ƣ�
��5��25��ʱ�й����ʵ��ܶȻ����£�Ksp[Mg��OH��
2]=5.61��10
-12��Ksp[Fe��OH��
3]=2.64��10
-3925��ʱ������Mg
2+��Fe
3+����Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH=8ʱ��c��Mg
2+����c��Fe
3+��=
��







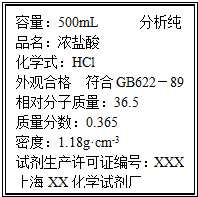 ȡ����Ϊ14.8g��Na2CO3��NaHCO3�Ĺ��������100mL 2.50mol?L-1������ǡ����ȫ��Ӧ���ٷų����壮
ȡ����Ϊ14.8g��Na2CO3��NaHCO3�Ĺ��������100mL 2.50mol?L-1������ǡ����ȫ��Ӧ���ٷų����壮