
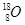 ��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣�
��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣� 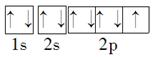 ��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣�
��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4s24p1 | B��3s23p2 | C��2s22p3 | D��2s23p4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ԣ�KOH��NaOH��LiOH |
| B�����ԣ�HNO3��H3PO4��HClO4 |
| C����ԭ�ԣ�Cl-��S2-��I- |
| D���ȶ��ԣ�H2O��HF��H2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| W | ԭ�ӵ��Ӳ���������������� |
| X | ԭ�Ӻ���L����s�ܼ��ĵ�������Ϊp�ܼ��ϵĵ���������һ�� |
| Y | Ԫ�ص�ԭ�Ӱ뾶�ڶ����������ϡ�������⣩ |
| Z | ԭ�Ӻ���p�ܼ��ϵĵ�����������s�ܼ��ϵĵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �������й�˵����ȷ����________����ѡ����ĸ����
�������й�˵����ȷ����________����ѡ����ĸ����| A��NaN3��KN3�ṹ���ƣ�ǰ�߾����ܽ�С |
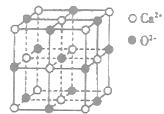
B������صľ����ṹ����ͼ��ʾ��ÿ�������з�̯2����ԭ�� |
| C�����ĵ�һ�����ܴ����� |
| D�����������º��ȶ�������Ϊ���ĵ縺��С |
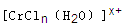 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��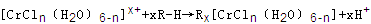
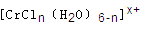 ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ������ĿΪ____ __________________.
������ĿΪ____ __________________. g/cm
g/cm �������ӵ�����Ϊ
�������ӵ�����Ϊ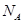 �����߳�a=________cm�����ú�
�����߳�a=________cm�����ú�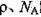 �ļ���ʽ��ʾ��
�ļ���ʽ��ʾ��
| A����һ�����ܴ�С��S>P>Si |
| B���縺��˳��C<N<O<F |
| C����Ϊ������Ca0��KCl�ߣ�����KCl��CaO�۵�� |
| D��SO2��CO2�Ļ�ѧ�������ƣ����ӽṹҲ����ֱ���ͣ���ͬ������SO2���ܽ�ȸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��������ܲ����ȫ������
��������ܲ����ȫ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com