
 �����£���0.1000mol?L-1 NaOH��Һ�ζ�20.00mL 0.1000mol?L-1������Һ���õζ�������ͼ������ͼ�еĢ١��ڡ��۵㣬������д��⣺
�����£���0.1000mol?L-1 NaOH��Һ�ζ�20.00mL 0.1000mol?L-1������Һ���õζ�������ͼ������ͼ�еĢ١��ڡ��۵㣬������д��⣺

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A���ÿ��淴Ӧ�Ļ�ѧ����ʽΪX��g��+Y��g��?2Z��g�� |
| B���ÿ��淴Ӧ�ġ�H��0 |
| C��ͼ1�з�Ӧ�ﵽƽ��ʱ��X��ת����Ϊ16.7% |
| D��T1��ʱ�����÷�Ӧ��ƽ�ⳣ��K=50����T1��T0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������¶� |
| B������ѹǿ |
| C�������¶�ͬʱ����N2 |
| D����ʱ��C02��N2�ӷ�Ӧ��ϵ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1�������������Դ���������ĵ缫��ӦʽΪ
��1�������������Դ���������ĵ缫��ӦʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |
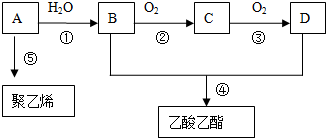
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
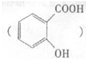 �ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�NaHCO3����Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⣮д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
�ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�NaHCO3����Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⣮д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڷ�Ӧ��NaClO3�ǻ�ԭ����Na2SO3�������� |
| B���з�Ӧ�ɵ�ClO2�������Դ���NaClO3�������� |
| C���÷�Ӧ�У�H2SO4�и�Ԫ�صĻ��ϼ�û�з����仯 |
| D��1mol NaClO3�μӷ�Ӧ������2mol����ת�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com