
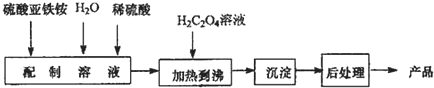
·ÖĪö £Ø1£©ČÜŅŗÖŠŃĒĢśĄė×ÓŗĶļ§øłĄė×ÓĖ®½ā£¬¼ÓČėÉŁĮæĮņĖį£¬ŅÖÖĘFe2+ŗĶNH4+Ąė×ÓĖ®½ā£»
£Ø2£©¢ŁBµć²ŠĮō¹ĢĢåÖŹĮæÕ¼Ōѳʷ×ÜÖŹĮæµÄ°Ł·ÖŹżĪ»80%£¬ĖšŹ§20%£¬FeC2O4.2H2OÖŠ½į¾§Ė®µÄÖŹĮæĪŖ$\frac{36}{180}$”Į100%=20%£¬¹ŹA”śB·¢Éś·“Ó¦ŹĒFeC2O4.2H2O¼ÓČČŹ§Č„½į¾§Ė®£»
¢ŚÓÉĶ¼æÉÖŖ¼ÓČČÉś³ÉCŹ±£¬·Ö½āĶźČ«£¬¼Ł¶Ø²ŻĖįŃĒĢś¾§ĢåĪŖ180g£¬ĘäĪļÖŹµÄĮæĪŖ1mol£¬ŌņC¹ĢĢåµÄÖŹĮæĪŖ180g”Į40%=72g£¬ĢśŌŖĖŲÖŹĮæĪŖ1mol”Į56g/mol=56£¬¹ŹÓ¦ŗ¬ÓŠŃõŌŖĖŲĪŖ72g-56g=16g£¬¼“ĢśŌ×ÓÓėŃõŌ×ÓĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬Ó¦ĪŖFeO£¬Bµćµ½CµćŹĒFeC2O4·Ö½āµÄ·“Ó¦£»
£Ø3£©øł¾ŻŹµŃéŌĄķ£¬2MnO4-+5H2C2O4+6H+ØT2Mn2++10CO2+8H2O£¬MnO4-+8H++5Fe2+ØT5Fe3++Mn2++4H2O£¬ĻČÓĆcmol/LøßĆĢĖį¼Ų±ź×¼ČÜŅŗµĪ¶ØѳʷČÜŅŗ£¬ŅņĪŖ²ŻĖįøłŗĶŃĒĢśĄė×Ó¶¼Äܱ»øßĆĢĖį¼Ų±ź×¼ČÜŅŗŃõ»Æ£¬ĖłŅŌµŚŅ»“ĪĻūŗÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖV1mLĪŖŃõ»Æ²ŻĖįøłŗĶŃĒĢśĄė×Ó¹²Ķ¬ĻūŗÄ£¬Č»ŗó¼ÓČė×ćĮæ Zn ·Ū£¬Ź¹ČÜŅŗÖŠµÄ Fe3+Ē”ŗĆČ«²æ»¹ŌĪŖ Fe2+£¬ŌŁÓĆc mol/L KMnO4ČÜŅŗµĪ¶ØøĆĀĖŅŗÖĮČÜŅŗ³öĻÖµ×ĻÉ«£¬ĻūŗÄKMnO4ČÜŅŗµÄĢå»żV2 mL¼“Ńõ»Æ¶ž¼ŪĢśµ„¶ĄĻūŗĵģ¬ĖłŅŌŃõ»Æ²ŻĖįøłĻūŗÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖ£ØV1-V2 £©mL£¬¾Ż“Ė·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ČÜŅŗÖŠŃĒĢśĄė×ÓŗĶļ§øłĄė×ÓĖ®¶¼ŹĒČõ¼īŃōĄė×Ó£¬Ė®½āĻŌĖįŠŌ£¬¼ÓČėÉŁĮæĮņĖį£¬ŅÖÖĘFe2+ŗĶNH4+Ąė×ÓĖ®½ā£¬
¹Ź“š°øĪŖ£ŗŅÖÖĘFe2+ŗĶNH4+Ąė×ÓĖ®½ā£»
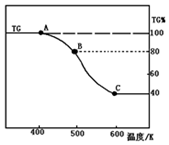
£Ø2£©¢ŁÓÉĶ¼æÉÖŖ£¬¼ÓČČÉś³ÉBŹ±Ź£Óą¹ĢĢåĪŖ80%£¬ĖšŹ§20%£¬FeC2O4.2H2OÖŠ½į¾§Ė®µÄÖŹĮæĪŖ$\frac{36}{180}$”Į100%=20%£¬¹ŹA”śB·¢Éś·“Ó¦ŹĒFeC2O4•2H2O¼ÓČČŹ§Č„½į¾§Ė®£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗFeC2O4•2H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$FeC2O4+2H2O£»
¹Ź“š°øĪŖ£ŗFeC2O4•2H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$FeC2O4+2H2O£»
¢ŚÓÉĶ¼æÉÖŖB¼ÓČČÉś³ÉCŹ±£¬·Ö½āĶźČ«£¬¼Ł¶Ø²ŻĖįŃĒĢś¾§ĢåĪŖ180g£¬ĘäĪļÖŹµÄĮæĪŖ1mol£¬ŌņC¹ĢĢåµÄÖŹĮæĪŖ180g”Į40%=72g£¬ĢśŌŖĖŲÖŹĮæĪŖ1mol”Į56g/mol=56£¬¹ŹÓ¦ŗ¬ÓŠŃõŌŖĖŲĪŖ72g-56g=16g£¬ŃõŌ×ÓĪļÖŹµÄĮæĪŖ1mol£¬¼“ĢśŌ×ÓÓėŃõŌ×ÓĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬Ó¦ĪŖFeO£¬ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦µē×ÓŹŲŗć·ÖĪö¼ĘĖć£¬Ģ¼ŌŖĖŲ»ÆŗĻ¼Ū“Ó+3¼Ū±ä»ÆĪŖ+2¼ŪŗĶ+3¼Ū£¬Éś³ÉŅ»Ńõ»ÆĢ¼ŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬Ō×ÓŹŲŗćÅäĘ½Š“³ö»Æѧ·½³ĢŹ½£»FeC2O4 $\frac{\underline{\;\;”÷\;\;}}{\;}$FeO+CO”ü+CO2”ü£¬
¹Ź“š°øĪŖ£ŗFeC2O4 $\frac{\underline{\;\;”÷\;\;}}{\;}$FeO+CO”ü+CO2”ü£»
£Ø3£©¢Ł²ŻĖįøł£ØC2O42-£©ÓėĖįŠŌøßĆĢĖį¼ŲČÜŅŗ·“Ó¦£¬ĻÖĻóŹĒÓŠĘųÅŻ²śÉś£¬×ĻÉ«ĻūŹ§£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2MnO4-+5H2C2O4+6H+ØT2Mn2++10CO2+8H2O£¬
¹Ź“š°øĪŖ£ŗ2MnO4-+5H2C2O4+6H+ØT2Mn2++10CO2+8H2O£»
¢Śøł¾ŻŅŌÉĻ·ÖĪö£¬ČōŹ”ĀŌ²½Öč¢ō£¬ŌņŹ¹¶ž¼ŪĢśĄė×ÓĘ«ÉŁ£¬ĖłŅŌĻūŗÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żŅ²V2 Ę«Š”£¬ŌņŃõ»Æ²ŻĖįøłĻūŗÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖ£ØV1-V2 £©mLĘ«“ó£¬ĖłŅŌ²ā¶ØµÄ²ŻĖįøłĄė×Óŗ¬ĮæŅ²Ę«“󣬹Ź“š°øĪŖ£ŗĘ«“ó£»
¢Ūøł¾ŻŅŌÉĻ·ÖĪö£¬Ńõ»Æ²ŻĖįøłĻūŗÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖ£ØV1-V2 £©mL£¬ÓÖ2MnO4-+5H2C2O4+6H+ØT2Mn2++10CO2+8H2O£¬ĖłŅŌmgѳʷ֊²ŻĖįøłĄė×ÓµÄĪļÖŹµÄĮæĪŖc£ØV1-V2£©”Į10-3”Į$\frac{5}{2}$”Į$\frac{100ml}{20ml}$=c£ØV1-V2£©”Į10-3”Į$\frac{25}{2}$mol£¬
¹Ź“š°øĪŖ£ŗc£ØV1-V2£©”Į10-3”Į$\frac{25}{2}$mol£®
µćĘĄ ±¾Ģāæ¼²é½ĻĪŖ×ŪŗĻ£¬Éę¼°µ½·½³ĢŹ½µÄŹéŠ“”¢ŹµŃ黳±¾²Ł×÷”¢µĪ¶ØµČ¼ĘĖć£¬×¢Ņā»ł“”ŹµŃéÖŖŹ¶µÄ»żĄŪ£¬°ŃĪÕŹµŃé²½Öč”¢ŌĄķŗĶ×¢ŅāŹĀĻīµČĪŹĢā£¬ĢāÄæÄѶČÖŠµČ£®


 æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Ļą¶Ō·Ö×ÓÖŹĮæ | ĆܶČ/£Øg•cm-3£© | ·Šµć/”ę | Ė®ÖŠČܽāŠŌ | |
| ŅģĪģ“¼ | 88 | 0.8123 | 131 | Ī¢ČÜ |
| ŅŅĖį | 60 | 1.0492 | 118 | ČÜ |
| ŅŅĖįŅģĪģõ„ | 130 | 0.8670 | 142 | ÄŃČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
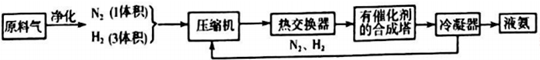
| ĘųĢå | CO | H2 | N2 | O2 |
| Ģå»ż£ØL£© | 25 | 60 | 15 | 2.5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| T/”ę | »īŠŌŃõ°Ł·Öŗ¬Įæ | ²śĀŹ |
| 5”«10 | 13.94 | 85.49 |
| 10”«15 | 14.02 | 85.78 |
| 15”«20 | 15.05 | 88.38 |
| 20”«25 | 14.46 | 83.01 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚ½ŗÓČėŗ£“¦Ņ׊Ī³ÉČż½ĒÖŽ | |
| B£® | AgNO3ČÜŅŗÖŠµĪČėĶ¬ÅضČNaClČÜŅŗ£¬Éś³É°×É«³Įµķ | |
| C£® | Ā±Ė®µć¶¹øÆ | |
| D£® | Ķ¬Ņ»øÖ±ŹĶ¬Ź±Ź¹ÓĆ²»Ķ¬ÅĘŗŵÄÄ«Ė®Ņ×·¢Éś¶ĀČū |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com