
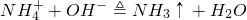
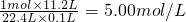
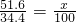 ��
��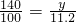 ��
��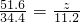


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | H+��Na+��Al3+��Ag+��Ba2+ |
| ������ | OH-��Cl-��CO32-��NO3-��SO42- |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?����һģ���Ȼ��У�C6H5CH2Cl����һ����Ҫ���л�����ԭ�ϣ��㷺Ӧ����ҽҩ��Ⱦ�ϡ������ϵ��л�������Ʒ���Ʊ����Ȼ���Ϊ��ɫҺ�壬����ǿ�ҵĴ������ã��е�Ϊ179.4�棬������ˮ���ɻ������Ҵ����ȷµȶ����л��ܼ�����ҵ�ϲ��üױ�����������ڹ��������·�Ӧ�ϳ��Ȼ��У�Ϊ̽����ͬ��ǿ�����Ժϳɲ��ʵ�Ӱ�죬��Ҫ�Բ�ͬ��ǿ�����µĴֲ�Ʒ���д��ȼ�⣮ÿһ���ⰴ�����²�����У�
��2011?����һģ���Ȼ��У�C6H5CH2Cl����һ����Ҫ���л�����ԭ�ϣ��㷺Ӧ����ҽҩ��Ⱦ�ϡ������ϵ��л�������Ʒ���Ʊ����Ȼ���Ϊ��ɫҺ�壬����ǿ�ҵĴ������ã��е�Ϊ179.4�棬������ˮ���ɻ������Ҵ����ȷµȶ����л��ܼ�����ҵ�ϲ��üױ�����������ڹ��������·�Ӧ�ϳ��Ȼ��У�Ϊ̽����ͬ��ǿ�����Ժϳɲ��ʵ�Ӱ�죬��Ҫ�Բ�ͬ��ǿ�����µĴֲ�Ʒ���д��ȼ�⣮ÿһ���ⰴ�����²�����У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�038
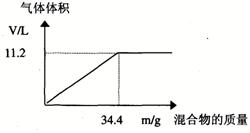
��100mL NaOH��Һ�м���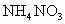 ��
��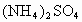 �Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺

(1)NaOH�����ʵ���Ũ��Ϊ_______________________��
(2)��NaOH�ܵ����Ϊ140mL�����������������51.6gʱ����ַ�Ӧ��������������(��״��)Ϊ______________L��
(3)��NaOH��Һ�����Ϊ180mL���������������Ϊ51.6gʱ����ַ�Ӧ��������������(��״��)______________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��100mL NaOH��Һ�м���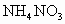 ��
�� �Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
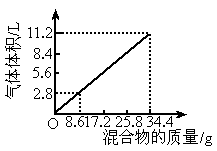
(1)NaOH�����ʵ���Ũ��Ϊ_______________________��
(2)��NaOH�ܵ����Ϊ140mL�����������������51.6gʱ����ַ�Ӧ��������������(��״��)Ϊ______________L��
(3)��NaOH��Һ�����Ϊ180mL���������������Ϊ51.6gʱ����ַ�Ӧ��������������(��״��)______________L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com